Polysialic acid lipid grafted derivatives and applications thereof
A technology of polysialic acid and derivatives, which is applied in the field of preparation and modification of microparticle preparations, can solve the problems that PEGylated macromolecules are not easy to be taken up by tumor cells, hinder the interaction between drugs and tumor cells, and block uptake by cells, so as to achieve targeted High stability, high yield, and controllable degree of polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
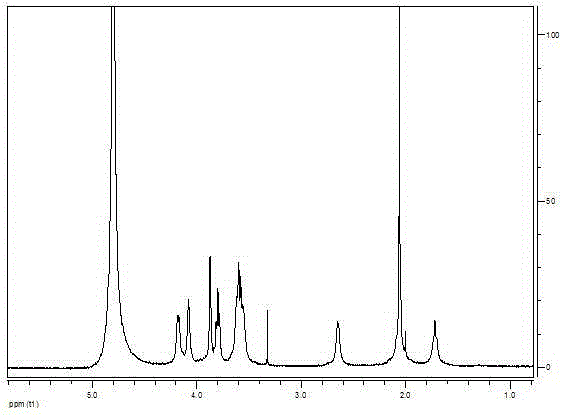
[0070] Embodiment 1: the synthesis of octadecyl dimethyl betaine BS18 and PSA
[0071] Dissolve BS18 (39.4mg, 0.01mmoL) in 4mL of FA, add EDC / NHS, and activate for 90min. At this time, the system is clear. PSA dissolved in 5mL of FA - Na + (60mg, 0.02mmoLSA monomer) was added to the reaction system, stirred at room temperature for 48h, the reaction solution was clear and slightly yellow.
[0072] Post-treatment: After diluting the substance in the dialysis bag (molecular weight cut-off 1000Da) to 40mL, the dialysis medium is ethanol-water (V / V, 1:2) system, with a volume of 2250mL for dialysis, dialysis at 60°C, and dialysis for 3 times within 4 hours Medium, after overnight dialysis, change the dialysis medium twice in the next 8 hours, and then overnight, a total of 48 hours, the material in the dialysis bag is clarified throughout the process. Part of the water-ethanol was spun out in a vacuum, and freeze-dried to obtain a white flocculent substance, which was the synthet...
Embodiment 2 18
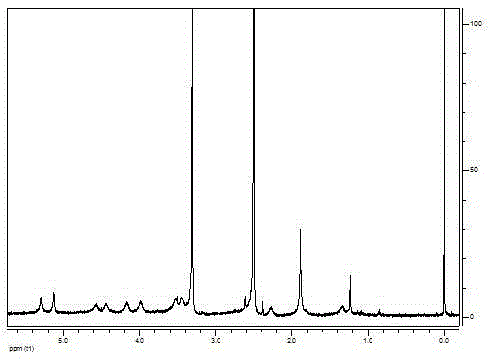
[0081] Embodiment 2. octadecanoic acid (OSA) and PSA synthesis
[0082] 3g H-type cation exchange resin, add 15mL containing 5g TBAB aqueous solution, stir at room temperature for 2h, pass the stirred suspension through the column, wash with deionized water, and mix the obtained cation exchange resin with PSA - Na + (200mg, 0.65mmoL) deionized aqueous solution was mixed and stirred overnight at room temperature. Take out the stirred liquid, centrifuge, collect the centrifuged liquid and freeze-dry, and the obtained substance is PSA - TBA + , with a solubility of 4mg PSA - TBA + / 1mL DMF.
[0083] Use IFS-55 Fourier transform infrared spectrometer (Bruker company, Switzerland) to obtain product PSA - TBA + Infrared analysis is carried out, and the test spectrum is shown in the accompanying drawing: Attached Figure 6 for PSA - TBA + . In the IR spectrum, while relative to PSA, it is at 2918.1, 2849.8cm -1 The stretching vibration peak of the alkyl chain is strengthe...
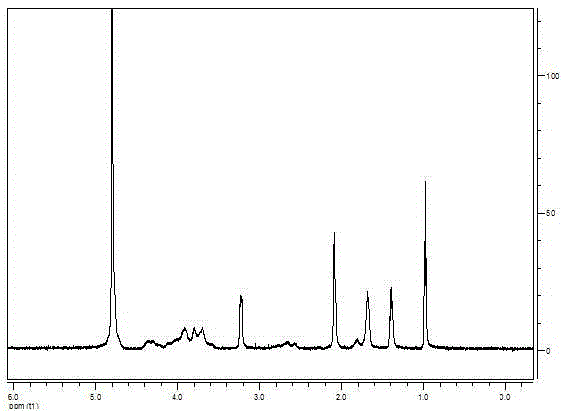
Embodiment 3
[0092] The mensuration of embodiment 3.PSA derivative critical micelle concentration (CMC)
[0093] Since the molecular structure of PSA-BS18 has hydrophilic groups and lipophilic groups, as a polymer block, it can spontaneously form micelles in water, and its critical micelle concentration can be determined by fluorescent probe method.
[0094] Precisely pipette 0.1mL with a concentration of 1×10 -5 Several parts of M-pyrene working solution were placed in the vials, blown dry with nitrogen gas, accurately weighed several parts of PSA-BS18, put them in the above-mentioned vials, and added 10 mL of pure water respectively to obtain the concentration of the pyrene working solution as 10 -7 M (the saturated solubility of pyrene in water is 7×10 -7 M), water bath ultrasonic 30min, place overnight, promptly obtain concentration and be respectively 5 * 10 -4 , 1×10 -3 , 3×10 -3 , 5×10 -3 , 1×10 -2 , 3×10 -2 , 5×10 -2 , 1×10 -1 , 5×10 -1 , 1,5g / L PSA-BS18 solution. The aq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com