A kind of hydrophobic polycaprolactone and preparation method thereof
A technology of polycaprolactone and hydrophobic type, which is applied in the field of hydrophobic polycaprolactone and its preparation, can solve the problems of difficult unsaturated monomer modification, poor carbon-bromine bond activity, low liquid repellency, etc., and achieve molecular weight High, narrow molecular weight distribution, the effect of avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
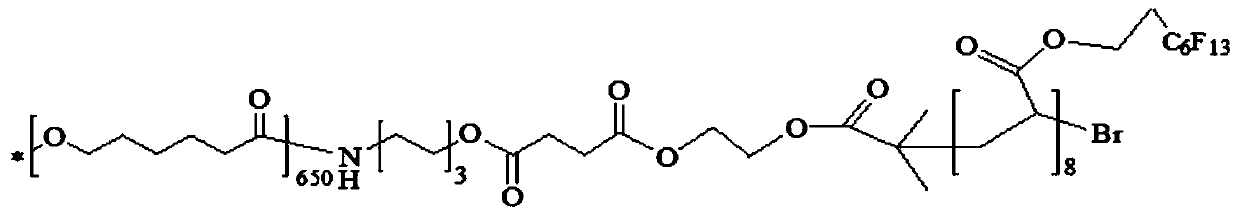
Embodiment 1
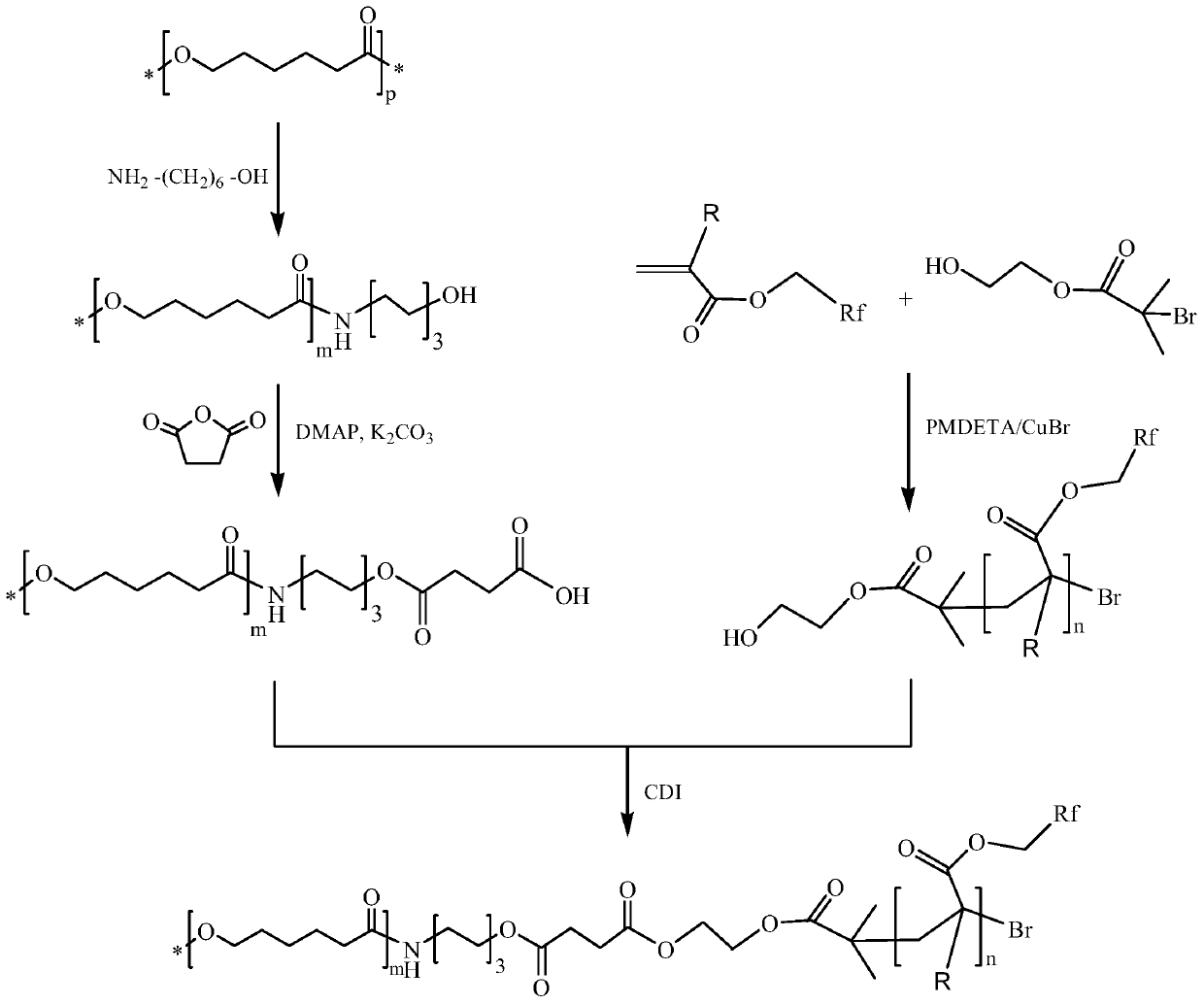
[0064] (1) Polycaprolactone terminal hydroxylation
[0065] Dissolve 50.0g of PCL with a molecular weight of 80,000 in 500g of 1,4-dioxane at 37°C. Under nitrogen protection, add 51g of 6-amino-1-hexanol and react for 8h. After the reaction was completed, the reaction solution was slowly added into 1000 g of absolute ethanol which was continuously stirred, and a solid was precipitated. After filtration, the filter cake was washed with absolute ethanol (100 g x 3 times), and dried in vacuum at 37° C. for 24 hours to obtain 39.6 g of the product with a yield of 79.2%. The molecular weight of the product was measured to be 69800.
[0066] (2) Polycaprolactone terminal carboxylation
[0067] Add 25.0 g of hydroxyl-terminated polycaprolactone (PCL-OH) and 28.5 g of succinic anhydride prepared above into 500 g of 1,4-dioxane. After stirring and dissolving, add 9.85g anhydrous potassium carbonate (K 2 CO 3 ) and 8.70g 4-dimethylaminopyridine (DMAP), reacted at room temperature f...
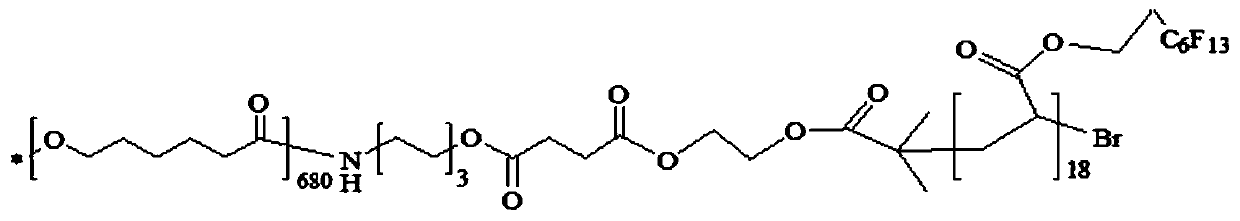
Embodiment 2
[0075] (1) Polycaprolactone terminal hydroxylation
[0076] Dissolve 50.0g of PCL with a molecular weight of 80,000 in 520g of 1,4-dioxane at 37°C, and add 49g of 6-amino-1-hexanol under nitrogen protection, and react for 12 hours. After the reaction was completed, the reaction solution was slowly added into 1050 g of absolute ethanol which was constantly stirred, and a solid was precipitated. After filtration, the filter cake was washed with absolute ethanol 100g × 3 times, and dried in vacuum at 37°C for 24h to obtain 37.9g of the product with a yield of 75.8%. The molecular weight of the product was measured to be 67500.
[0077] (2) Polycaprolactone terminal carboxylation
[0078] Add 25.0 g of hydroxyl-terminated polycaprolactone (PCL-OH) and 29.2 g of succinic anhydride prepared above to 510 g of 1,4-dioxane. After stirring and dissolving, add 9.88g anhydrous potassium carbonate (K 2 CO 3) and 8.75g 4-dimethylaminopyridine (DMAP), reacted at room temperature for 4...
Embodiment 3
[0086] (1) The operation steps of polycaprolactone terminal hydroxylation and polycaprolactone terminal carboxylation are the same as in Example 1.
[0087] (2) Preparation of hydroxyl-terminated poly(1H,1H,2H,2H-tridecafluorooctyl acrylate) by ATRP method
[0088] Dissolve 418ug of ethylene glycol bromoisobutyrate and 620ug of pentamethyldiethylenetriamine (PMDETA) in 60g of 2-butanone, after dissolving, add 0.6g of cuprous bromide, under nitrogen protection at 40°C The reaction was stirred for 15 minutes to obtain a catalyst. Add 65.6g of 1H,1H,2H,2H-tridecafluorooctyl acrylate (TFOA), heat to 80°C, and react for 6 hours. After the reaction was completed, 360g THF and 120g benzotrifluoride were added and passed through a neutral alumina (200-300 mesh) column to obtain a pale yellow clear solution. The solution was evaporated under reduced pressure at 65°C to remove the solvent, then the crude product was added to 1050g of anhydrous methanol, and a solid was precipitated, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com