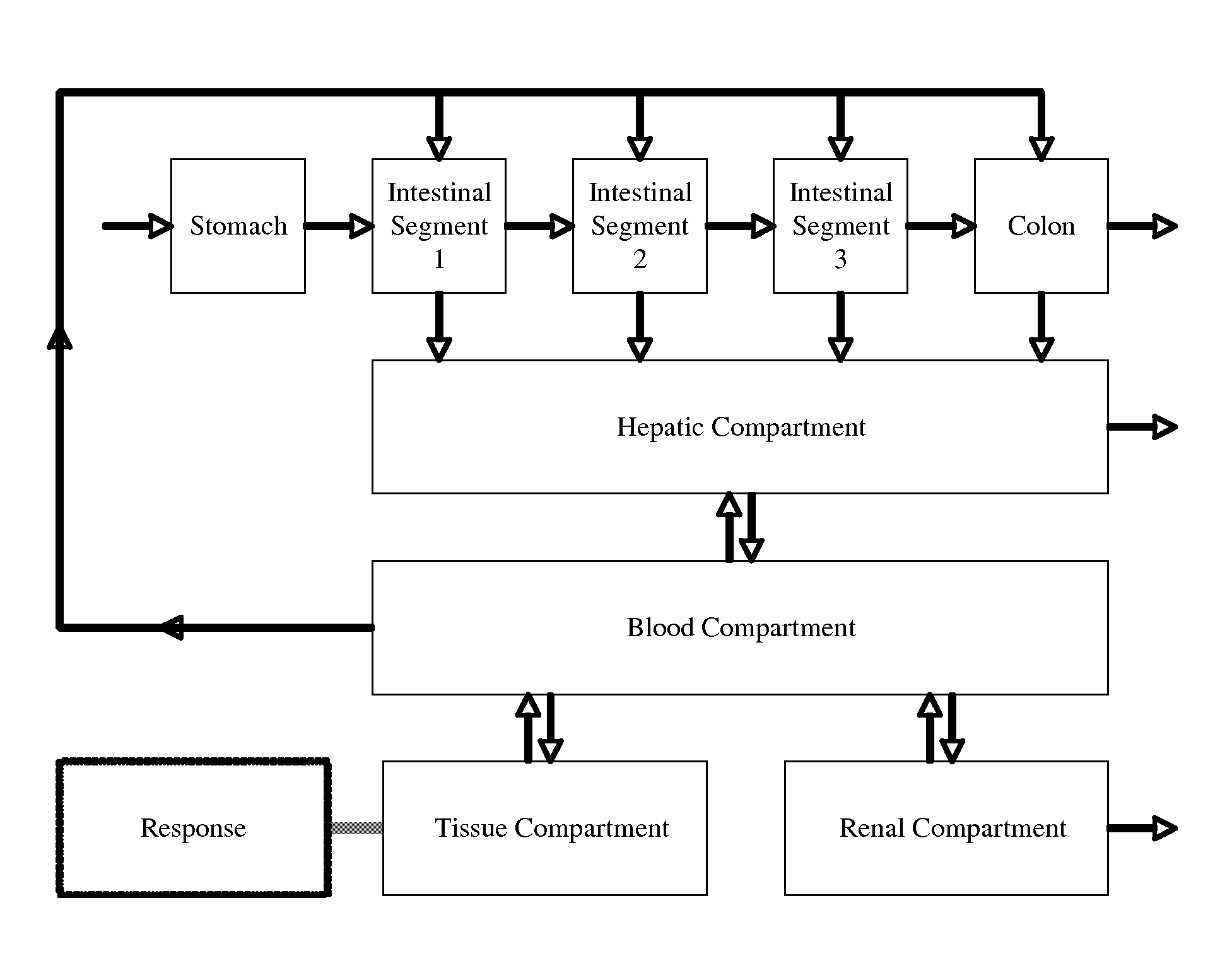
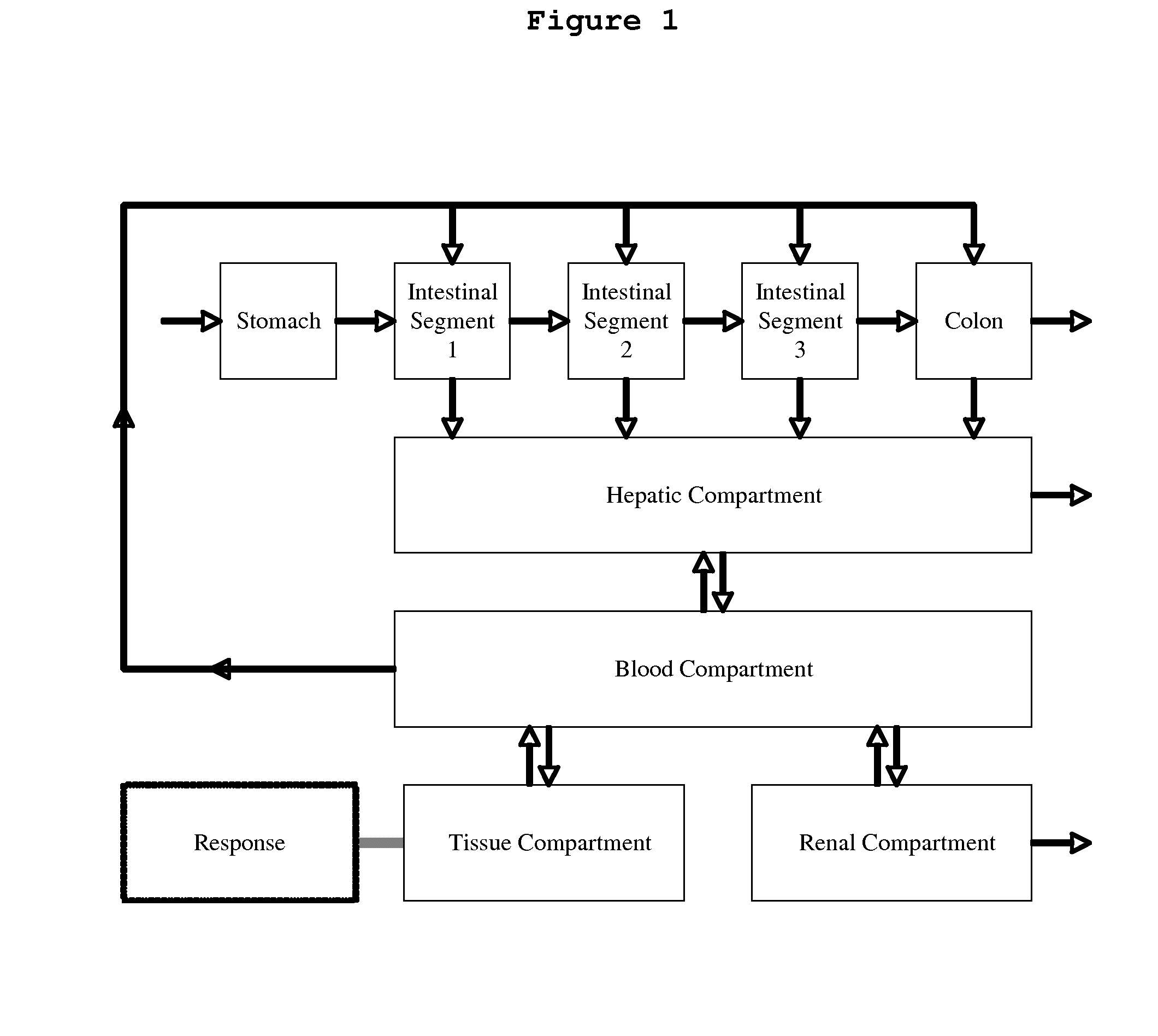
Pharmaceutical Platform Technology for the Development of Natural Products
a technology of pharmaceutical platform and natural product, applied in the direction of instruments, chemical property prediction, biocide, etc., can solve the problems of no de novo combinatorial compound approved as a drug, low activity of ginsenosides, and complex research in this field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Approach to Model Development for Estimating the Contribution of Each Component to the Response of a Mixture
[0084]The objective of this example is to establish a mathematical framework upon which a mathematical model is developed to describe and quantify activity of individual components in a mixture. The mathematical problem that arises can be formulated as follows. Suppose one had a number of samples of the same herbal preparation (for example, Panax ginseng) coming from different sources, each of which has a potentially different composition in terms of quantity of active components. Suppose the samples are labeled by an index “i” that runs from 1 to M. Suppose also that each sample contains N active ingredients labeled by index “j” that runs from 1 to N. The concentration of each ingredient can be determined and is denoted as c(i,j) such that summing c(i,j) from 1 to N over j gives 1 (or 100% as they all add up to the total amount in each sample) for all samples (denoted by i's)...
example 2
Construction of a Model to Describe the Activity of Individual Components in a Mixture
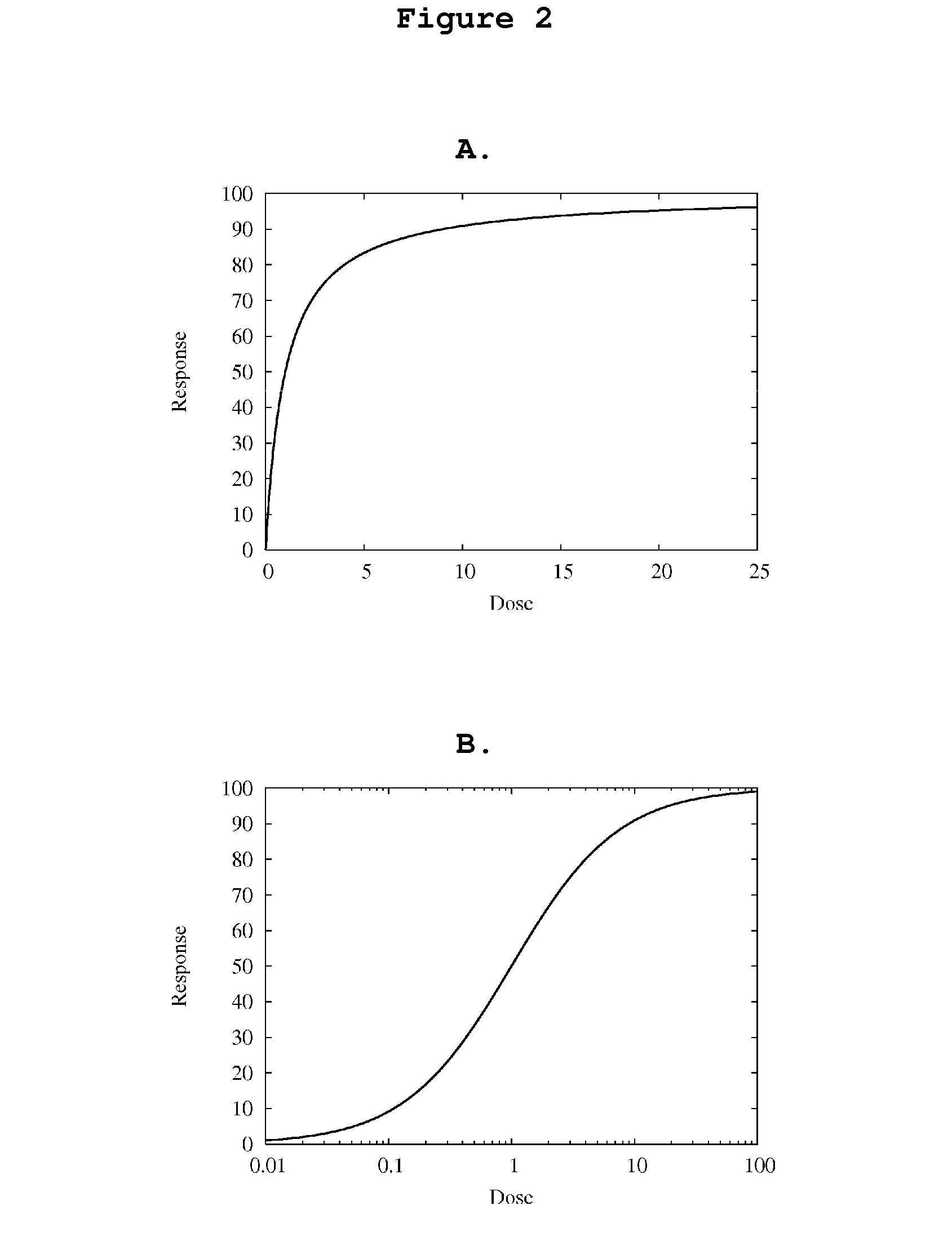
[0088]The objective of this example is to employ the approach described in Example 1 to construct a model to describe individual component activity in a mixture. This model will be used to estimate activity of individual components of a hypothetical mixture with pre-determined activities.
[0089]The physiological reality of a dose-response relationship is that at low doses, responses are proportional to dose. However, response reaches a limit at higher doses. In this example, the Michaelis-Menton equation:
R=Rmax·CEC50+C(4)
Where R is response, Rmax is maximum response, C is dose or concentration, and EC50 is C that elicits 50% of Rmax, is used to model this type of dose-response behavior (FIG. 2). To facilitate modeling, R is linearized using the following equation (FIG. 4):
r=R1-R(5)
[0090]In constructing the model, it is convenient to shift from considering the dose of a mixture, to considering the do...
example 3
Detailed Approach in Identifying Active Components and the Interacting Species
[0100]The objective of this example is to outline an approach to mine all the active and interacting components in a mixture. In an herbal extract, there may exist hidden unknowns that have not been previously identified. This may occur when the components are transparent to quantitative or qualitative analysis; for example, components may have very little UV absorbance when a UV detector is used for identifying individual components. This aspect makes the problem open-ended from the point of view of model development and refinement in the course of experimentation.
[0101]This problem was looked at from a couple of different angles stated below. 1) It could be determined that by accounting for all the known variables, it is still not possible to describe the activity properly which will warrant additional empirical studies of the composition. 2) It is possible to assume that unknowns always exist and they c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| least absolute shrinkage | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com