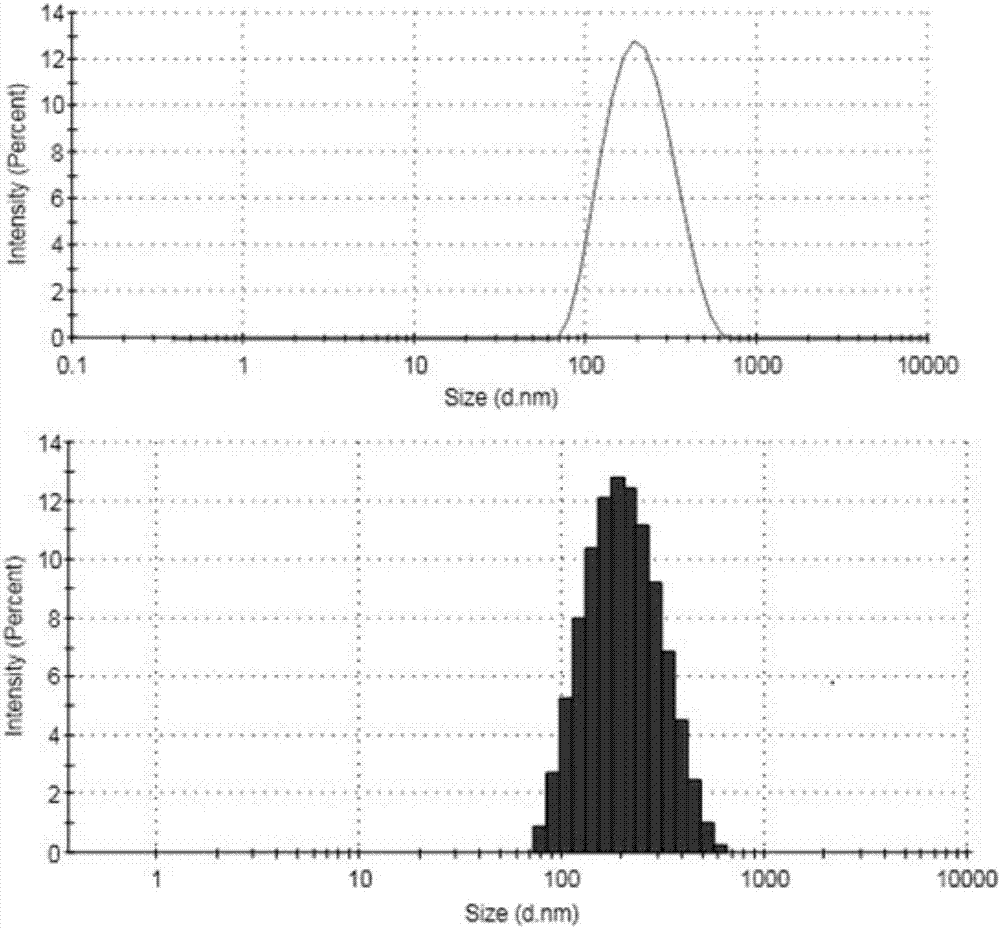
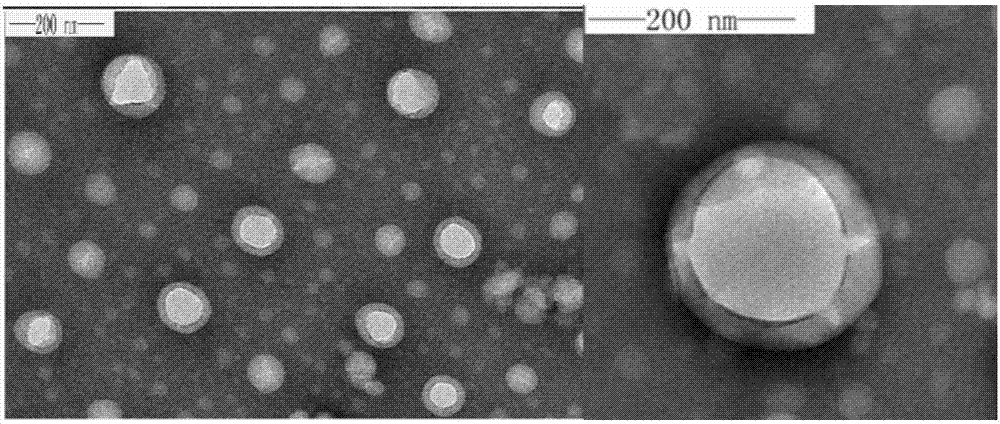
Polymeric micelle capable of realizing synchronous administration of sorafenib and curcumin and preparation method of polymeric micelle
A kind of polymer glue and polymer technology, which is applied in the direction of medical preparations, drug combinations, and pharmaceutical formulas of non-active ingredients, can solve the problems of no medicine available, and can not further expand the application range, etc., to achieve strong therapeutic effect and prolong Retention time, the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. In vitro cell inhibitory activity of Sorafenib and curcumin raw materials in combination
[0030] (1) Solution configuration: Precisely weigh 60 mg of curcumin, 30 mg of sorafenib, 60 mg of curcumin plus 30 mg of sorafenib, and add 1 mL of dimethyl sulfoxide (DMSO) to three 10 mL plastic centrifuge tubes. , ultrasonically assisted dissolution, dissolved and moved into the ultra-clean bench, sucked 5 μL into 5mL culture medium respectively, filtered it with a 0.22 μm microporous membrane, used it as the original solution, and diluted it with the culture medium to 2 times as the different concentrations of drug-containing culture for MTT experiments base.
[0031] The final drug-containing medium concentration was:
[0032] a. Curcumin group: 60, 30, 15, 7.5, 3.75 μg / mL;
[0033] b. Sorafenib group: 30, 15, 7.5, 3.25, 1.875 μg / mL;
[0034] c. Combined medication group: curcumin concentration (μg / mL) / sorafenib concentration (μg / mL), 60 / 30, 30 / 15, 15 / 7.5,
[0035] 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com