Method for cancer treatment based on combination of drugs
A cancer treatment and drug delivery technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as the inability to administer drugs by systemic injection, and increase the range of selectivity and range Increased, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
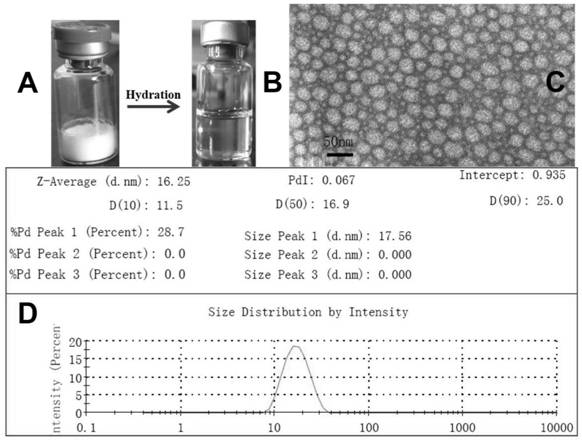
[0041] (1) MPEG-PDLLA and I3A are co-dissolved in 100mL of absolute ethanol according to a certain mass ratio (9g:1g), ultrasonically dissolved and added to a spray dryer, and dried quickly, the dried powder is collected, and 850ml of 14% (W / V) Aqueous solution of sucrose for injection, after shaking and ultrasonically dissolving, adjust the pH value to 5.0 with citric acid, add 14% (W / V) aqueous solution of sucrose for injection to 1000ml scale, pressurize and filter at 0.22um, divide the filtrate, 1ml / bottle, placed in a lyophilizer to quickly freeze to -40°C, pre-frozen for 4 hours, and then sublimated and dried to obtain I3A-PM lyophilized powder for future use. When in use, take 1 bottle of I3A-PM freeze-dried powder and add 2ml of water for injection or 0.9% sodium chloride water for injection or glucose water for injection, shake to dissolve, after the bubbles dissipate, draw out and add 500ml of normal saline or glucose water for injection, intravenously Infusion.
Embodiment 2
[0043] (2) MPEG-PCL and I3A are co-dissolved in 100mL of absolute ethanol according to a certain mass ratio (9g:1g), ultrasonically dissolved and added to a spray dryer, dried quickly, and the dried powder is collected, and 850ml of 14% (W / V) Aqueous solution of sucrose for injection, after shaking and ultrasonically dissolving, adjust the pH value to 5.0 with citric acid, add 14% (W / V) aqueous solution of sucrose for injection to 1000ml scale, pressurize and filter at 0.22um, divide the filtrate, 1ml / bottle, placed in a lyophilizer to quickly freeze to -40°C, pre-frozen for 4 hours, and then sublimated and dried to obtain I3A-PM lyophilized powder for future use. When in use, take 1 bottle of I3A-PM freeze-dried powder and add 2ml of water for injection or 0.9% sodium chloride water for injection or glucose water for injection, shake to dissolve, after the bubbles dissipate, draw out and add 500ml of normal saline or glucose water for injection, intravenously Infusion.
Embodiment 3
[0045] (3) PDLLA-PEG-PDLLA and I3A were co-dissolved in 100mL of absolute ethanol according to a certain mass ratio (9g:1g), ultrasonically dissolved and added to a spray dryer, dried quickly, and the dried powder was collected, and 850ml of 14% ( W / V) sucrose aqueous solution for injection, after shaking and ultrasonically dissolving, adjust the pH value to 5.0 with citric acid, add 14% (W / V) sucrose aqueous solution for injection to 1000ml scale, filter under 0.22um pressure, and pack the filtrate separately, 1ml / bottle, placed in a lyophilizer and quickly frozen to -40°C, pre-frozen for 4 hours, sublimated and dried to obtain I3A-PM lyophilized powder for later use. When in use, take 1 bottle of I3A-PM freeze-dried powder and add 2ml of water for injection or 0.9% sodium chloride water for injection or glucose water for injection, shake to dissolve, after the bubbles dissipate, draw out and add 500ml of normal saline or glucose water for injection, intravenously Infusion. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com