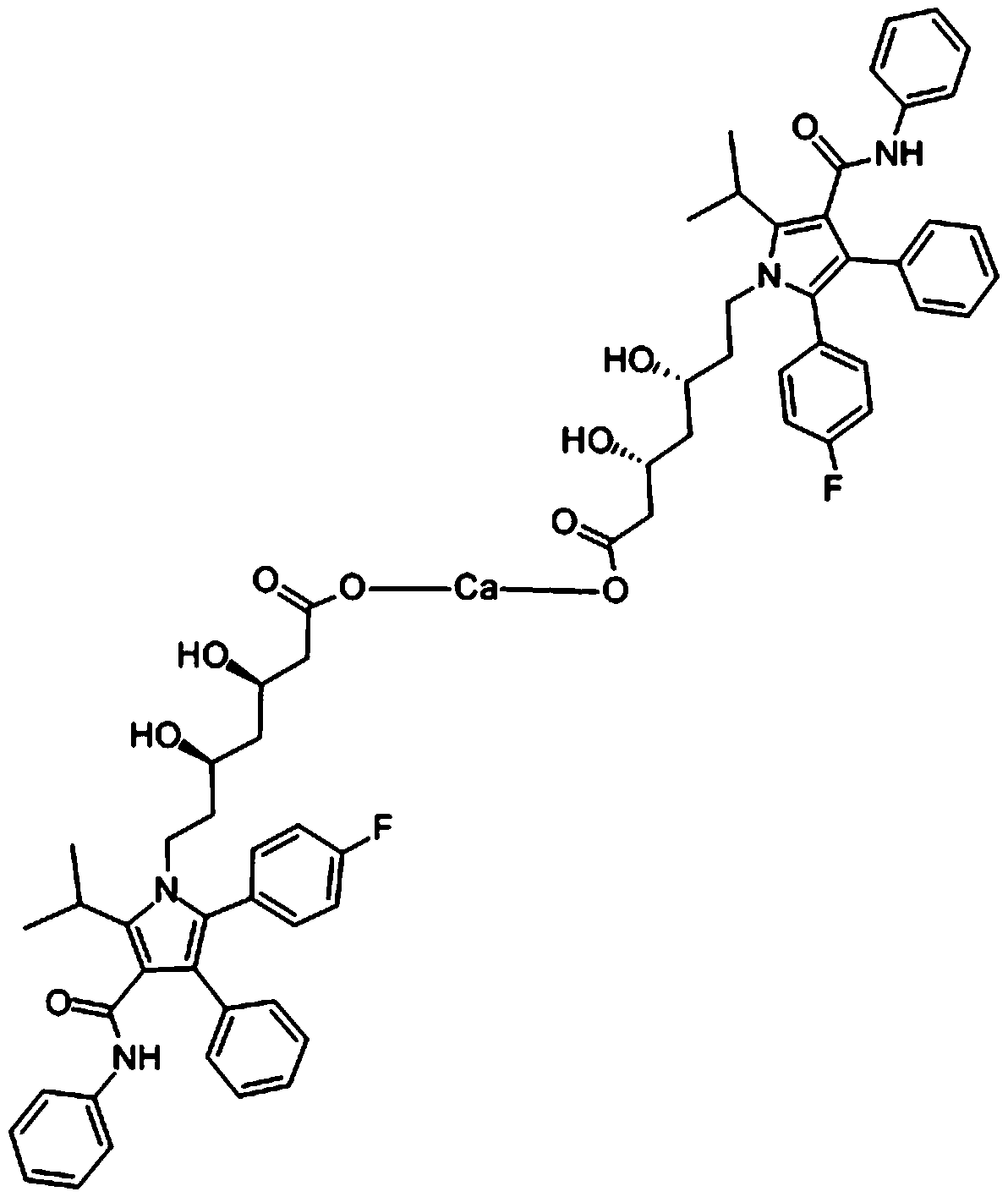
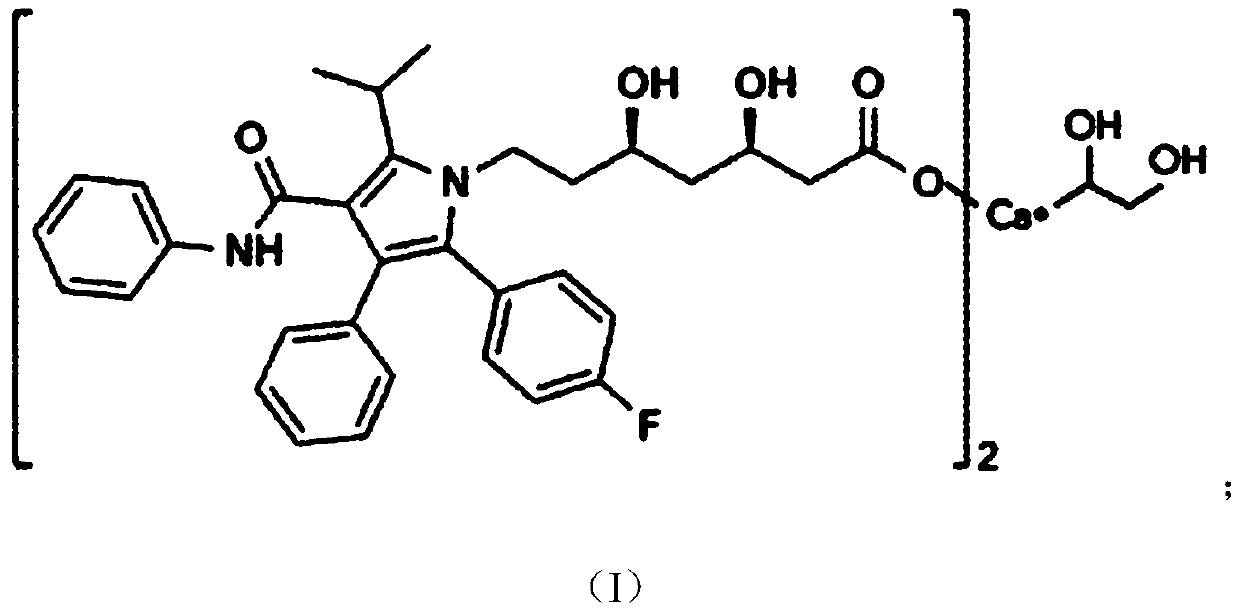
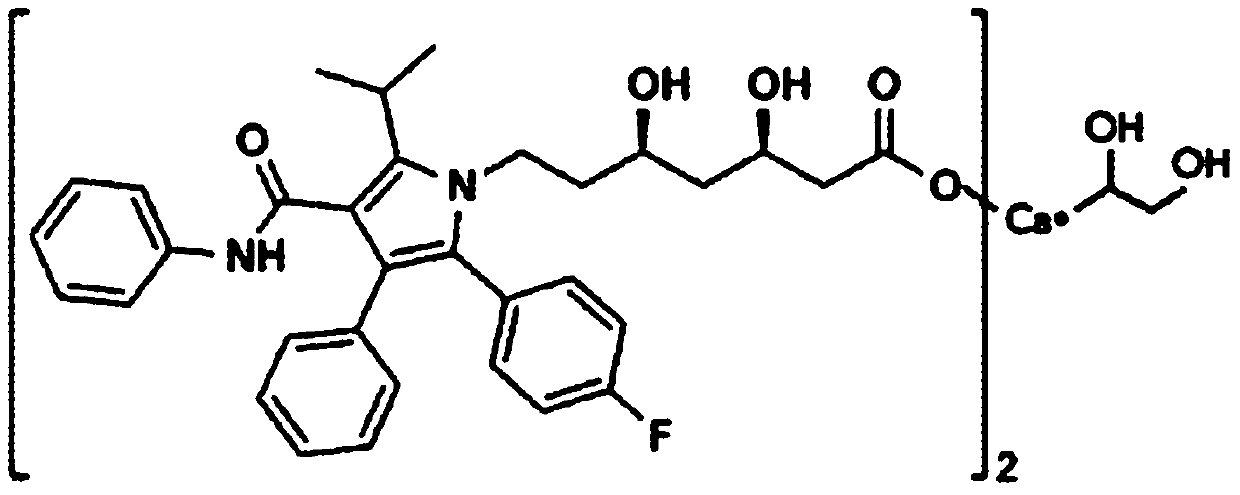
A kind of atorvastatin calcium tablet and preparation method thereof
A technology of atorvastatin calcium and tablet is applied in the field of atorvastatin calcium tablet and its preparation, which can solve the problems of air environmental factor sensitivity, harsh preparation processing conditions, unstable biological activity, etc., and achieves improved biological absorption. Availability, promoting drug disintegration, reducing the effect of interfacial free energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The atorvastatin calcium tablet of the present embodiment comprises the component of following raw material:
[0064] 1 part by weight of atorvastatin calcium, 10 parts by weight of a filler, 40 parts by weight of a disintegrant, 24 parts by weight of a binder, 8 parts by weight of a lubricant, and 10 parts by weight of a calcium fortifier;
[0065] Wherein, the calcium fortifier is calcium carbonate; the filler is lactose; the disintegrant is crospovidone; the binder is pregelatinized starch; and the lubricant is silicon dioxide.
[0066] Its preparation method is as follows:
[0067] (1) Get atorvastatin calcium, filler, disintegrating agent, binding agent by selected parts by weight, with 2% of the total consumption of described disintegrating agent and described atorvastatin calcium, filler, The binder is mixed evenly, water is used as the granulation solvent, and spray granulation is used for granulation to obtain atorvastatin calcium drug-containing granules;
...
Embodiment 2
[0071] The atorvastatin calcium tablet of the present embodiment comprises the component of following raw material:
[0072] 10 parts by weight of atorvastatin calcium, 80 parts by weight of a filler, 30 parts by weight of a disintegrant, 5 parts by weight of a binder, 15 parts by weight of a lubricant, and 20 parts by weight of a calcium fortifier;
[0073] Wherein, the calcium fortifier is calcium hydrogen phosphate; the filler is pregelatinized starch; the disintegrant is aluminum magnesium silicate; the binder is dextran; and the lubricant is stearin Magnesium acid.
[0074] Its preparation method is as follows:
[0075] (1) Get atorvastatin calcium, filler, disintegrating agent, binding agent by selected parts by weight, with 98% of the total consumption of described disintegrating agent and described atorvastatin calcium, filler, The binder is mixed evenly, and ethanol is used as the granulation solvent, and spray granulation is used for granulation to obtain atorvasta...
Embodiment 3
[0079] The atorvastatin calcium tablet of the present embodiment comprises the component of following raw material:
[0080] 20 parts by weight of atorvastatin calcium, 45 parts by weight of filler, 20 parts by weight of disintegrant, 40 parts by weight of binder, 1 part by weight of lubricant, and 1 part by weight of calcium fortifier;
[0081] Wherein, the calcium fortifier is calcium sulfate; the filler is hydroxypropyl methylcellulose; the disintegrant is pregelatinized starch; the binder is gelatin; the lubricant is talcum powder .
[0082] Its preparation method is as follows:
[0083] (1) Get atorvastatin calcium, filler, disintegrating agent, binding agent by selected parts by weight, with 50% of the total consumption of described disintegrating agent and described atorvastatin calcium, filler, The binder is mixed evenly, acetone is used as the granulation solvent, and spray granulation is used for granulation to obtain atorvastatin calcium drug-containing granules; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com