Novel ethylene diamine derivative
A crystal form, ethyl technology, applied in the preparation of carbamate derivatives, the preparation of amino compounds from amines, the preparation of organic compounds, etc., can solve the problems of serious adverse reactions, optic nerve damage, low oral bioavailability, etc. Effects of improved bioavailability, overcoming severe obstacles, good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
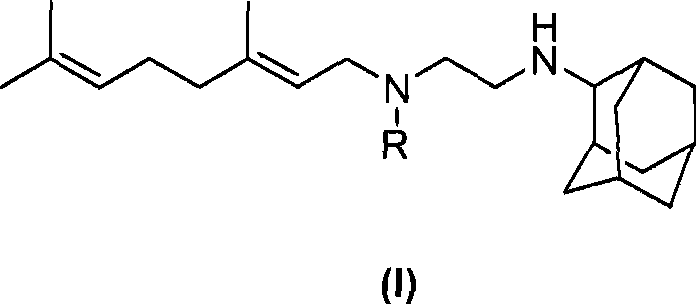
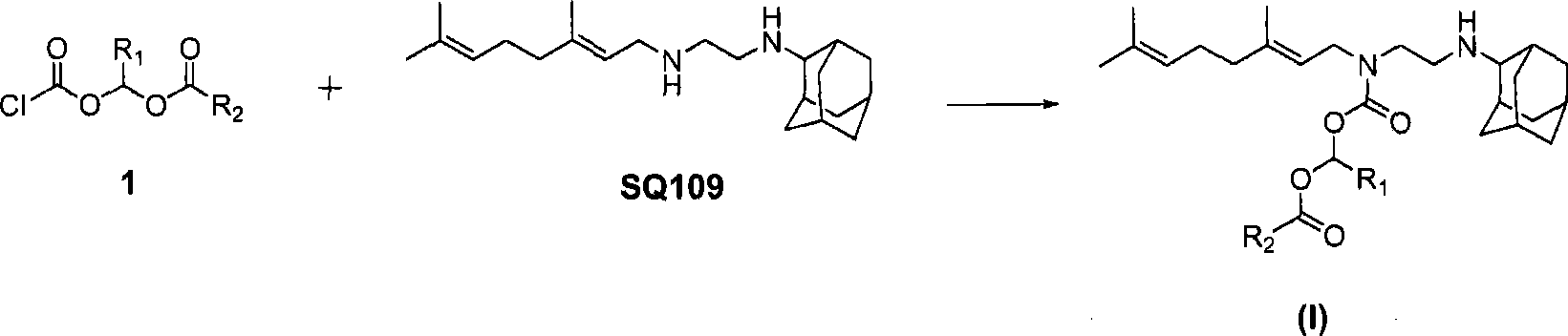
[0121] Example 1: (E)-N-(3,7-Dimethyl-octa-2,6-dienyl)-N-(2-(adamantan-2-ylamino)ethyl)-carbamic acid (pivaloyloxy)methyl ester (Compound I-a)
[0122] method one
[0123] (E)-N-(3,7-Dimethyl-octa-2,6-dienyl)-N'-(adamantan-2-yl)-ethane-1,2-diamine (700mg, 2.12mmol) was dissolved in 20mL THF, added triethylamine (642mg, 6.36mmol) and cooled to 0°C in an ice bath, then added dropwise (pivaloyloxy)methyl chloroformate (413mg, 2.12mmol), and recovered after dropping Stir at room temperature for 2h. Add 75 mL of water, extract with ethyl acetate (50 mL×2), wash with saturated brine, and dry over sodium sulfate. The desiccant was filtered off, concentrated, and column chromatography (petroleum ether: ethyl acetate = 3:1) gave a light yellow liquid (543 mg, yield 52.5%).
[0124] 1 H NMR (400MHz CDCl 3 )δ: 1.18(s, 9H), 1.42-1.47(m, 3H), 1.58(s, 3H), 1.62-1.70(m, 9H), 1.72-1.84(m, 6H), 1.85-2.08(m, 6H), 2.60-2.75(m, 3H), 3.28(t, J=6.59Hz, 1H), 3.36(t, J=6.59Hz, 1H), 3.88(d, J=6...
Embodiment 2
[0138] Example 2: (E)-N-(3,7-Dimethyl-oct-2,6-dienyl)-N-(2-(adamantan-2-ylamino)ethyl)-carbamic acid (pivaloyloxy)methyl ester maleate (compound I-b)
[0139] Compound I-a (250 mg) was dissolved in 5 mL of isopropanol, and maleic acid (59 mg) was added. Stir at room temperature for 0.5 h, and concentrate to give a white solid (310 mg, yield 100%).
[0140] Mp: 164-166°C; 1 H NMR (400MHz DMSO) δ: 1.44(s, 9H), 1.56-1.74(m, 15H), 1.84-2.09(m, 12H), 3.01-3.09(m, 2H), 3.45-3.51(m, 3H) , 3.83-3.90(m, 2H), 5.06-5.11(m, 2H), 5.71(s, 2H), 6.03(s, 2H), 8.31(brs, 2H); MS(ESI): 489
Embodiment 3
[0141] Example 3: (E)-N-(3,7-Dimethyl-oct-2,6-dienyl)-N-(2-(adamantan-2-ylamino)ethyl)-carbamic acid (pivaloyloxy)methyl ester mesylate (compound I-c)
[0142] Compound I-a (250 mg) was dissolved in 5 mL of isopropanol, and methanesulfonic acid (49 mg) was added. Stir at room temperature for 0.5 h, and concentrate to obtain a white solid (293 mg, yield 98%).
[0143] Mp: 173-175°C; MS (ESI): 489
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com