Salts prepared from 2-(1-acyloxy-n-amyl)benzoic acid and basic amino acid or aminoguanidine, and preparation method and application thereof
A technology of aminoguanidine benzoate and acetoxy-n-pentyl, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as poor chemical stability, achieve excellent water solubility, excellent pharmacokinetic properties, and enhance therapeutic effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
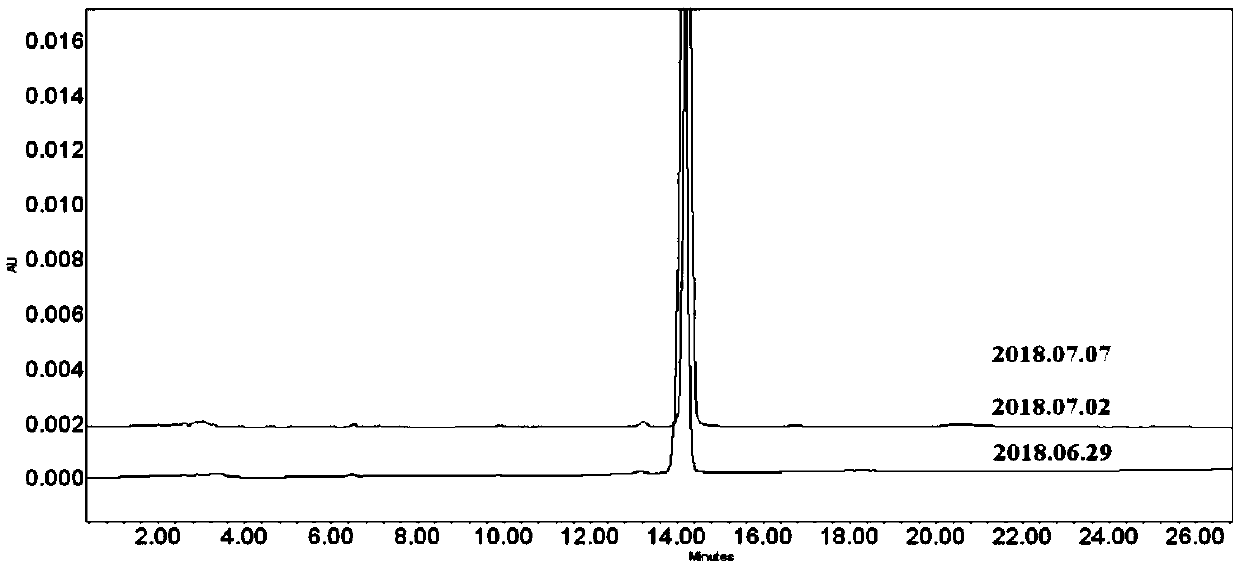
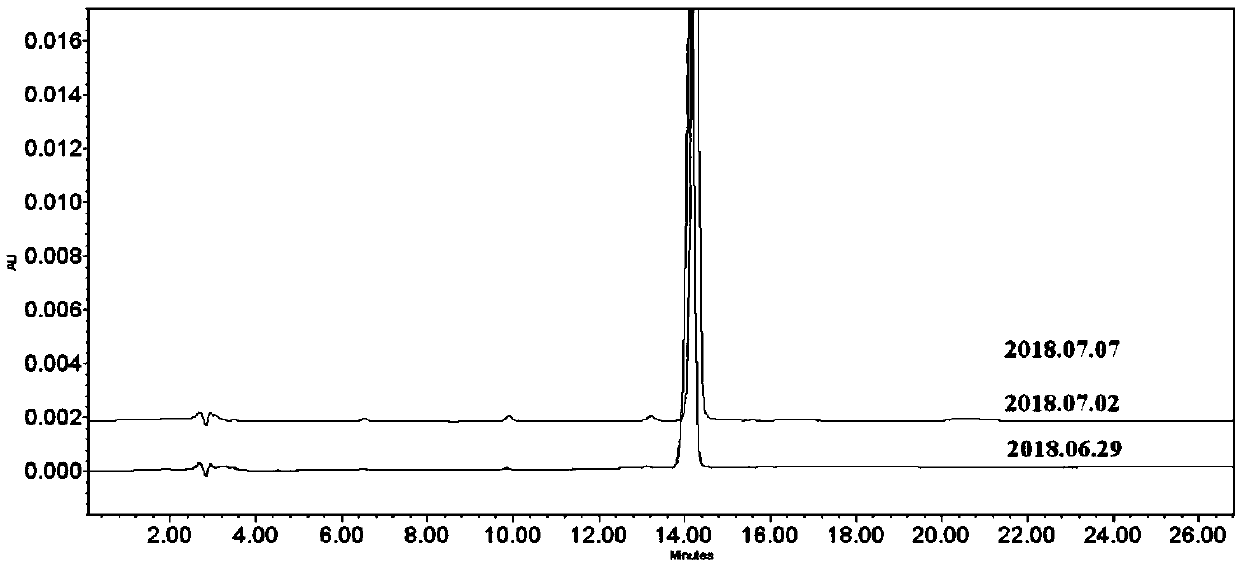
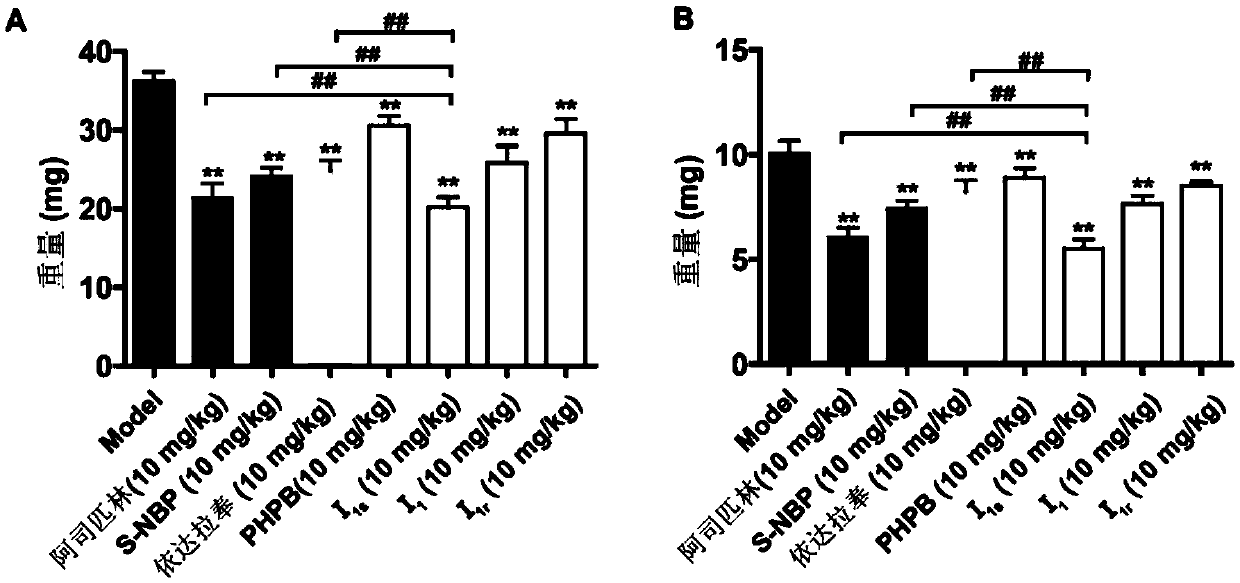
Image
Examples
Embodiment 1
[0067] Embodiment 1: (R / S)-2-(1-acetoxy n-pentyl) benzoic acid L-arginine salt (I 1 ) preparation
[0068] Under the condition of -30~-5℃, to the ether (300mL) solution containing 10.9g (52.6mmol) (R / S)-2-(1-hydroxy-n-pentyl)benzoic acid (II), add 21.8mL (157.8mmol) of triethylamine, 0.6g (5.2mol) of DMAP, then slowly dropwise added 11.1mL (157.8mmol) of acetyl chloride, and stirred for 5 hours. After the reaction, add about 35mL10% hydrochloric acid, acidify to pH 2–3, stir for 2 hours, separate the organic layer, anhydrous Na 2 SO 4 Drying, filtration, concentration under reduced pressure, and column chromatography [petroleum ether: ethyl acetate (v:v) = 10:1] gave 9.96 g of (R / S)-2-(1-acetoxy) in the form of white needle crystals n-pentyl)benzoic acid, yield 76%, mp:65–66°C; MS(m / z):249[M 1 –H] - ; 1 H NMR (300MHz, CDCl 3 ):δ(ppm):0.97(t, 3H,CH 3 , J=6.9Hz), 1.38–1.52 (m, 4H, C H 2 C H 2 CH 3 ), 1.84–1.99 (m, 2H, CHC H 2 CH 2 ),2.17(s,3H,CHOCOC H 3 ),6.69...
Embodiment 2
[0070] Embodiment 2: (R / S)-2-(1-propionyloxy-n-pentyl) benzoic acid L-arginine salt (I 2 ) preparation
[0071] Under the condition of -30~-5℃, to the ether (300mL) solution containing 9.96g (39.8mmol) (R / S)-2-(1-hydroxy-n-pentyl)benzoic acid (II), add 21.8ml (157.8mmol) of triethylamine, 0.6g (5.2mmol) of DMAP, then slowly dropwise added 13.1mL (157.8mmol) of propionyl chloride, and stirred for 5 hours. After the reaction, add about 35mL10% hydrochloric acid, acidify to pH 2–3, stir for 2 hours, separate the organic layer, anhydrous Na 2 SO 4Dry, filter, concentrate under reduced pressure, and then go through column chromatography [petroleum ether: ethyl acetate (v:v) = 10:1] to obtain 9.0 g of oily (R / S)-2-(1-propionyloxynormal Amyl) benzoic acid, yield 65%; MS (m / z): 263 [M 1 –H] - ; 1 H NMR (300MHz, CDCl 3 ):δ(ppm):0.96(t,3H,CH 2 CH 2 C H 3 ,J=6.9Hz), 1.21(t,3H,COCH 2 C H 3 ,J=7.8Hz),1.32–1.51(m,4H,C H 2 C H 2 CH 3 ),1.80–1.98(m,2H, CHC H 2 CH 2 ),2.4...
Embodiment 3
[0073] Embodiment 3: (R / S)-2-(1-n-butyryloxy-n-pentyl) benzoic acid L-arginine salt (I 3 ) preparation
[0074] Under the condition of -30~-5℃, to the ether (300mL) solution containing 9.96g (39.8mmol) (R / S)-2-(1-hydroxy-n-pentyl)benzoic acid (II), add 21.8mL (157.8mmol) of triethylamine, 0.6g (5.2mmol) of DMAP, and then slowly dropwise added 15.9mL (157.8mmol) of n-butyryl chloride, and stirred for 5 hours. Add about 35mL10% hydrochloric acid after the reaction, acidify to pH 2–3, stir for 2 hours, separate the organic layer, anhydrous Na 2 SO 4 Drying, filtration, concentration under reduced pressure, and column chromatography [petroleum ether: ethyl acetate (v: v) = 10:1] gave 8.4 g of oily (R / S)-2-(1-n-butyryloxy-n- Pentyl)benzoic acid, yield 58%; MS(m / z): 277[M 1 –H] - ; 1 H NMR (300MHz, CDCl 3 ):δ(ppm):0.89–1.06(m,6H,2×CH 3 ), 1.33–1.51 (m,4H,CH 2 C H 2 C H 2 CH 3 ),1.7-1.78(m,2H,COCH 2 C H 2 CH 3 ),1.85–1.98 (m,2H,CHC H 2 CH 2 ),2.41(t,2H,COC H 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com