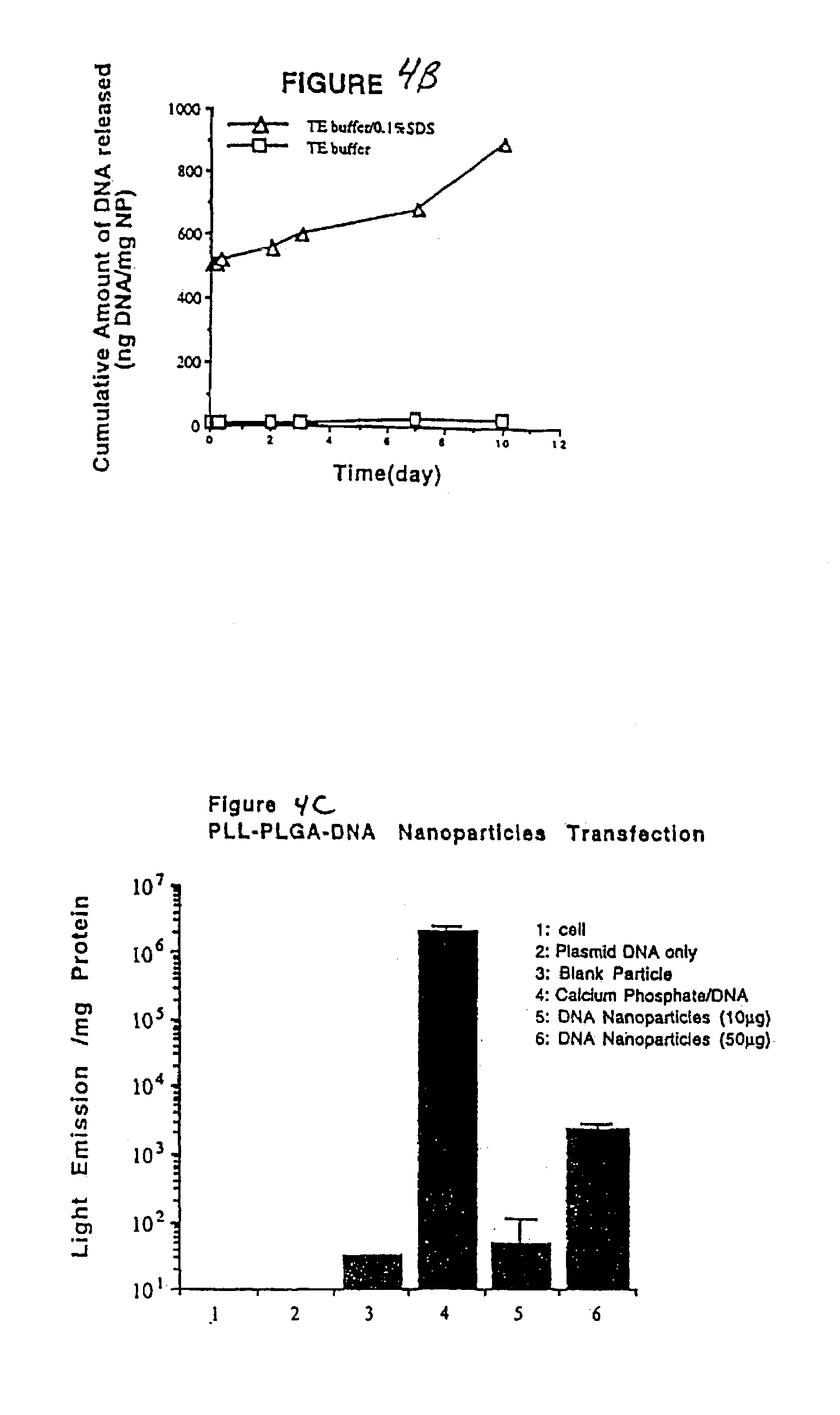
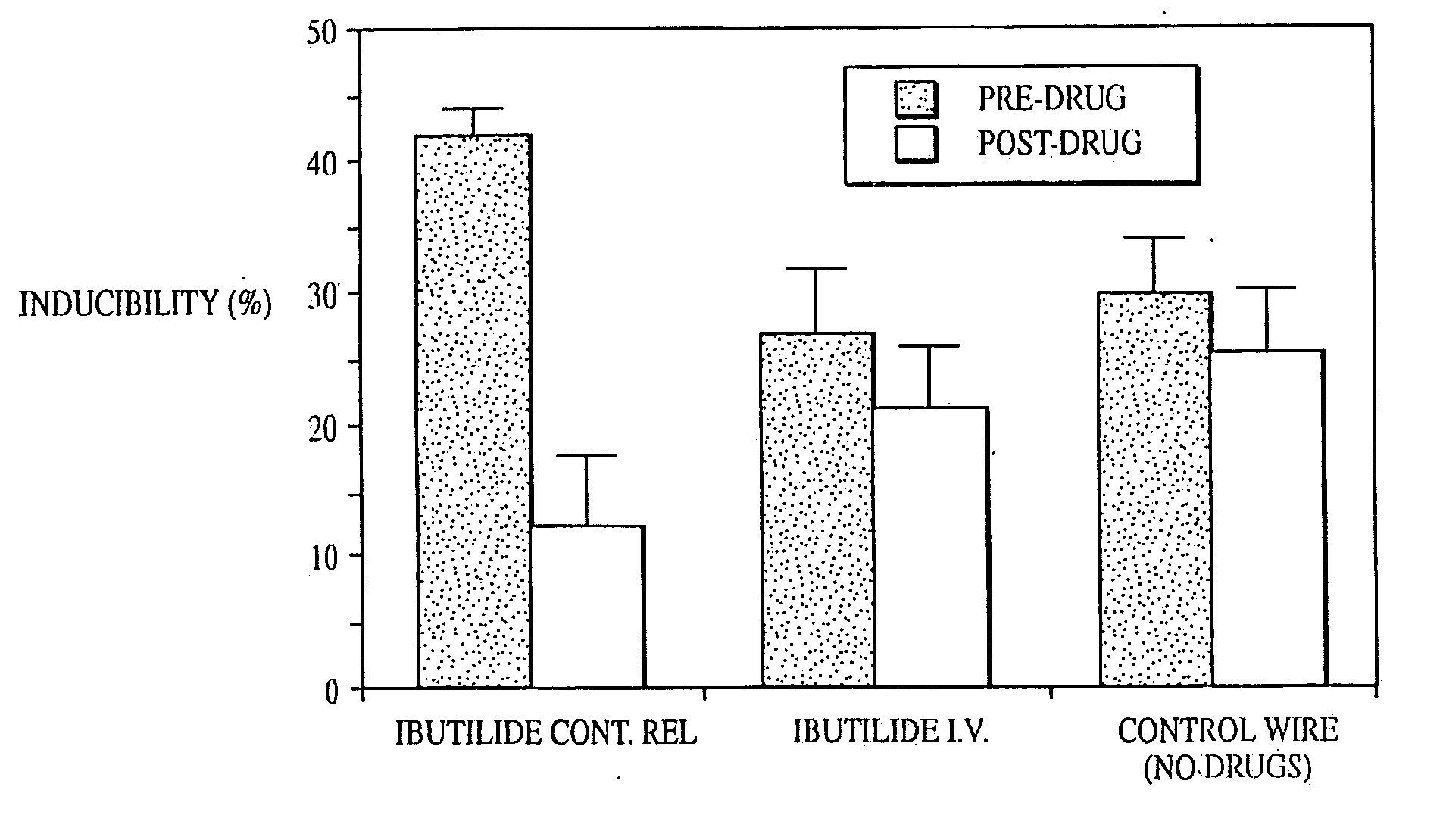
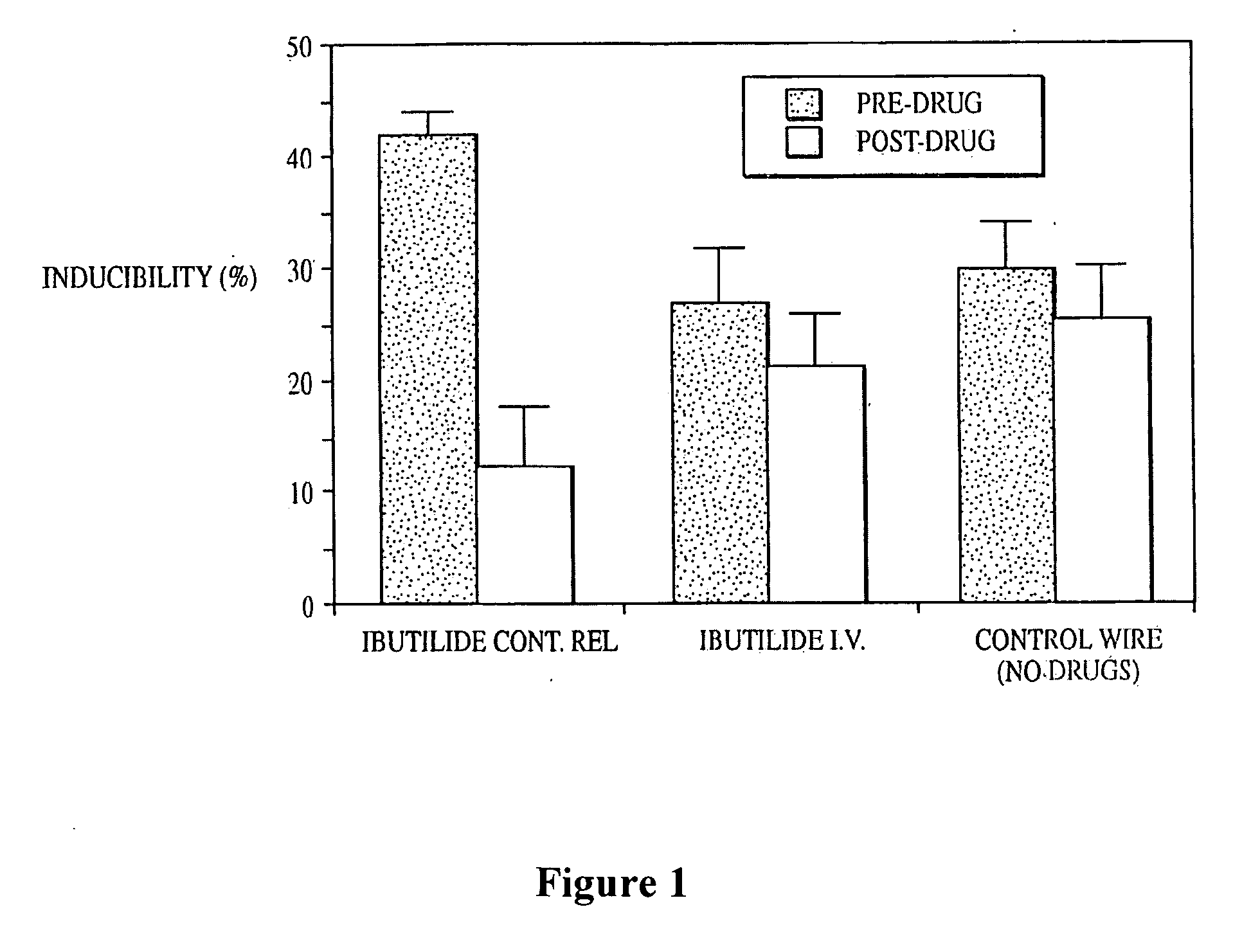
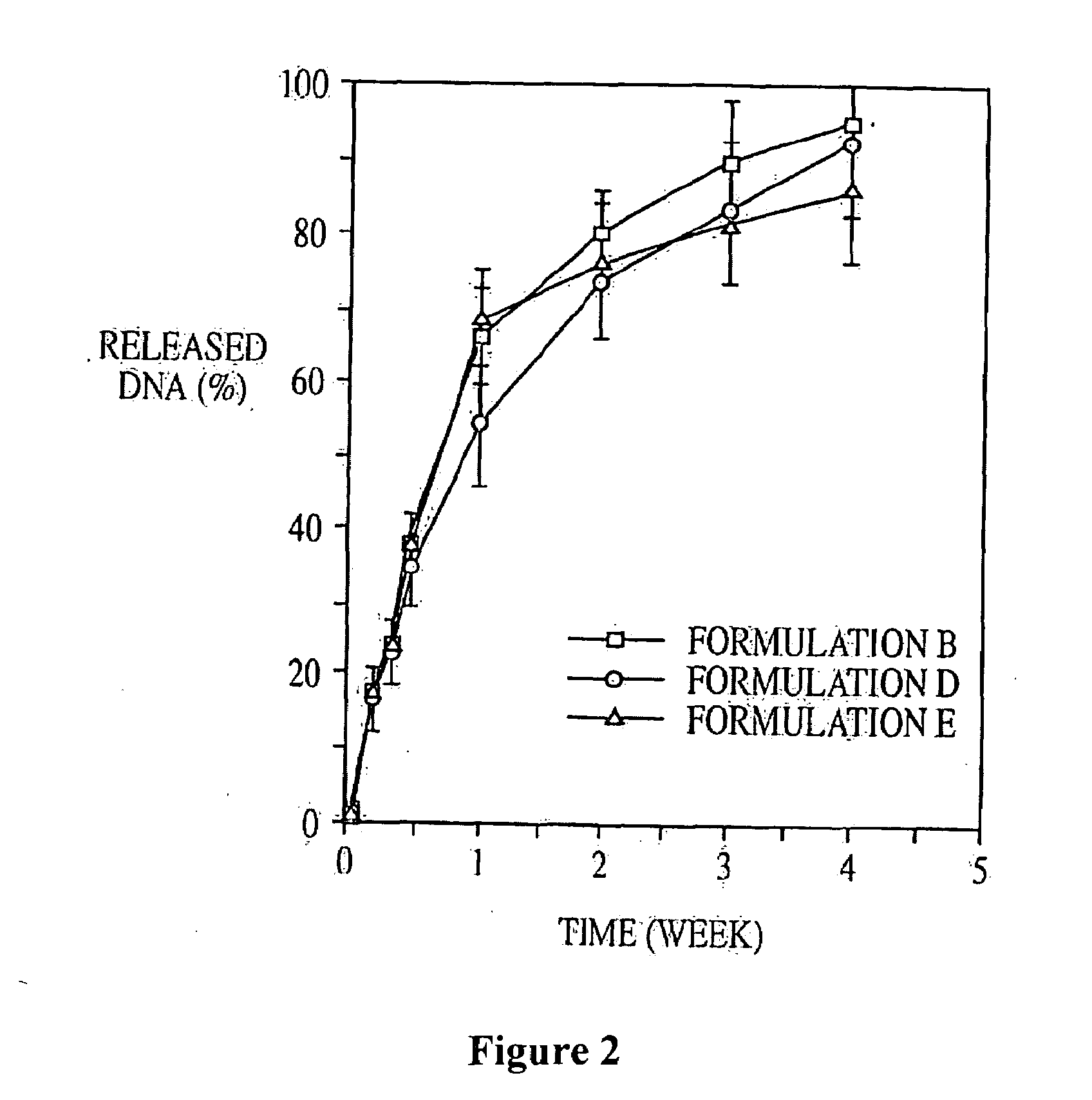
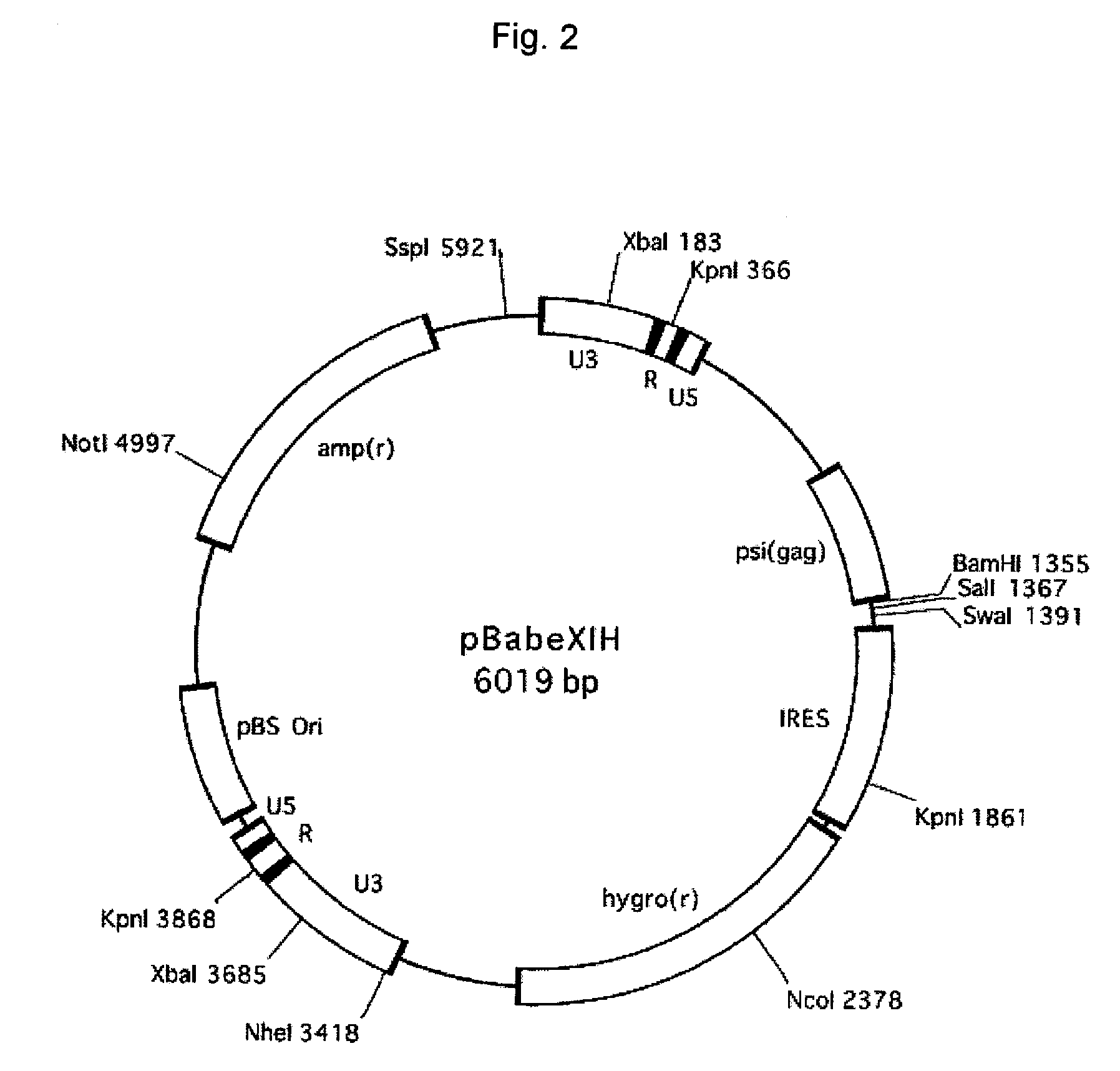
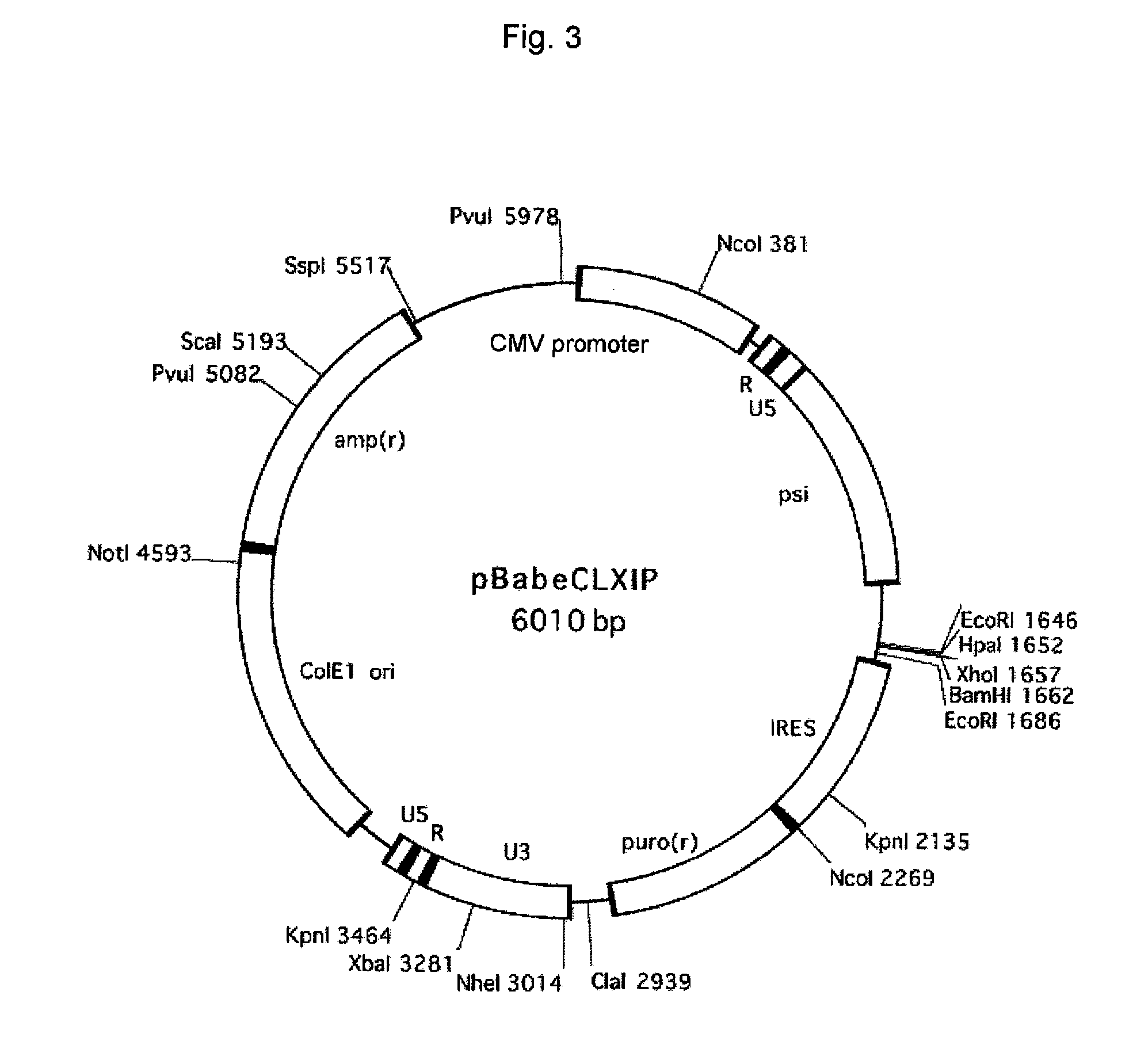
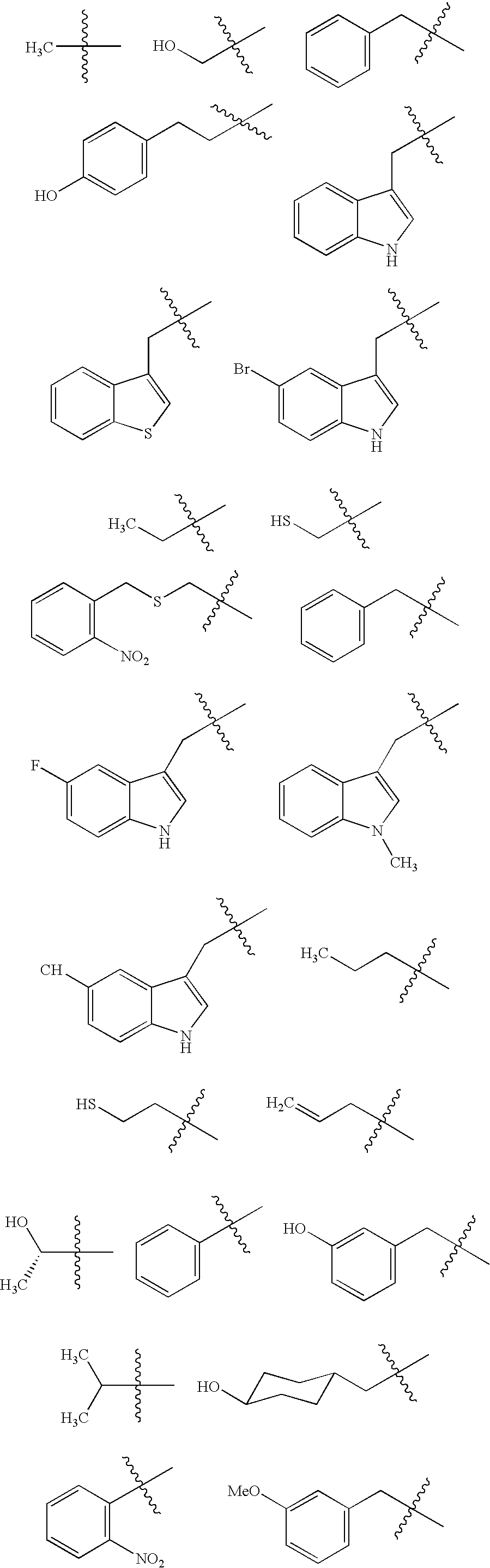
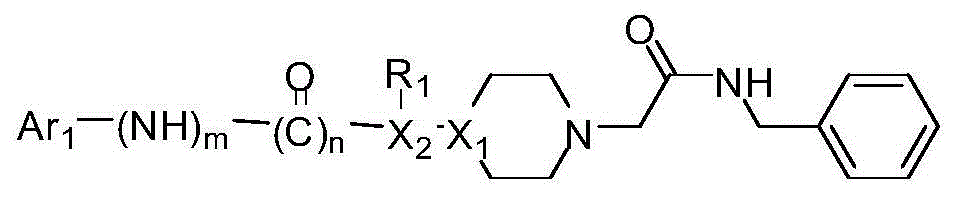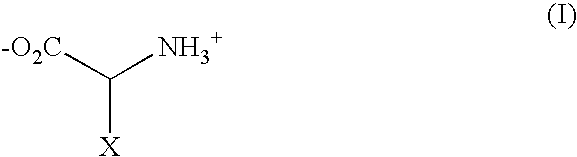Patents
Literature
55 results about "HUMAN ETHER-A-GO-GO-RELATED GENE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
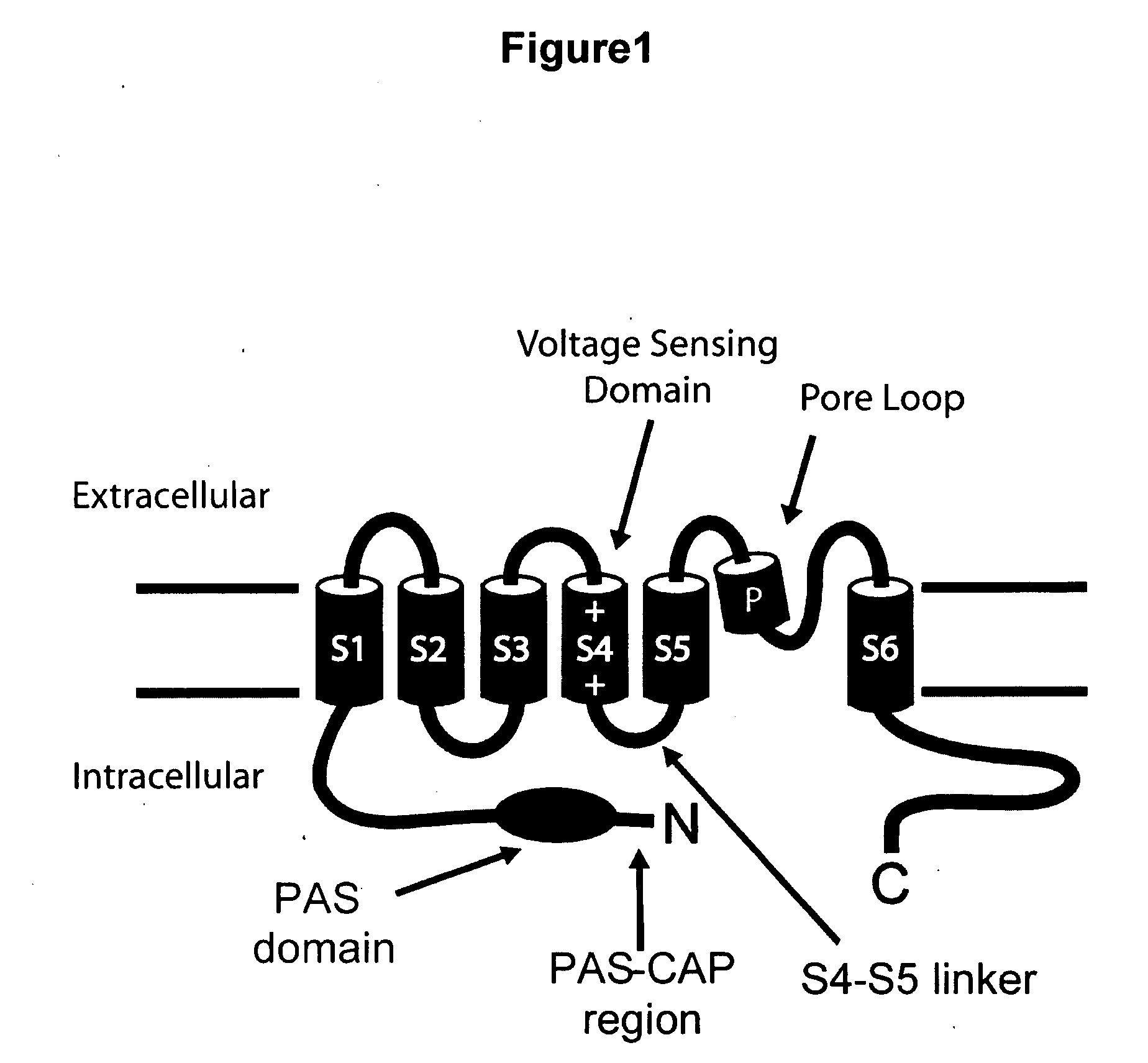
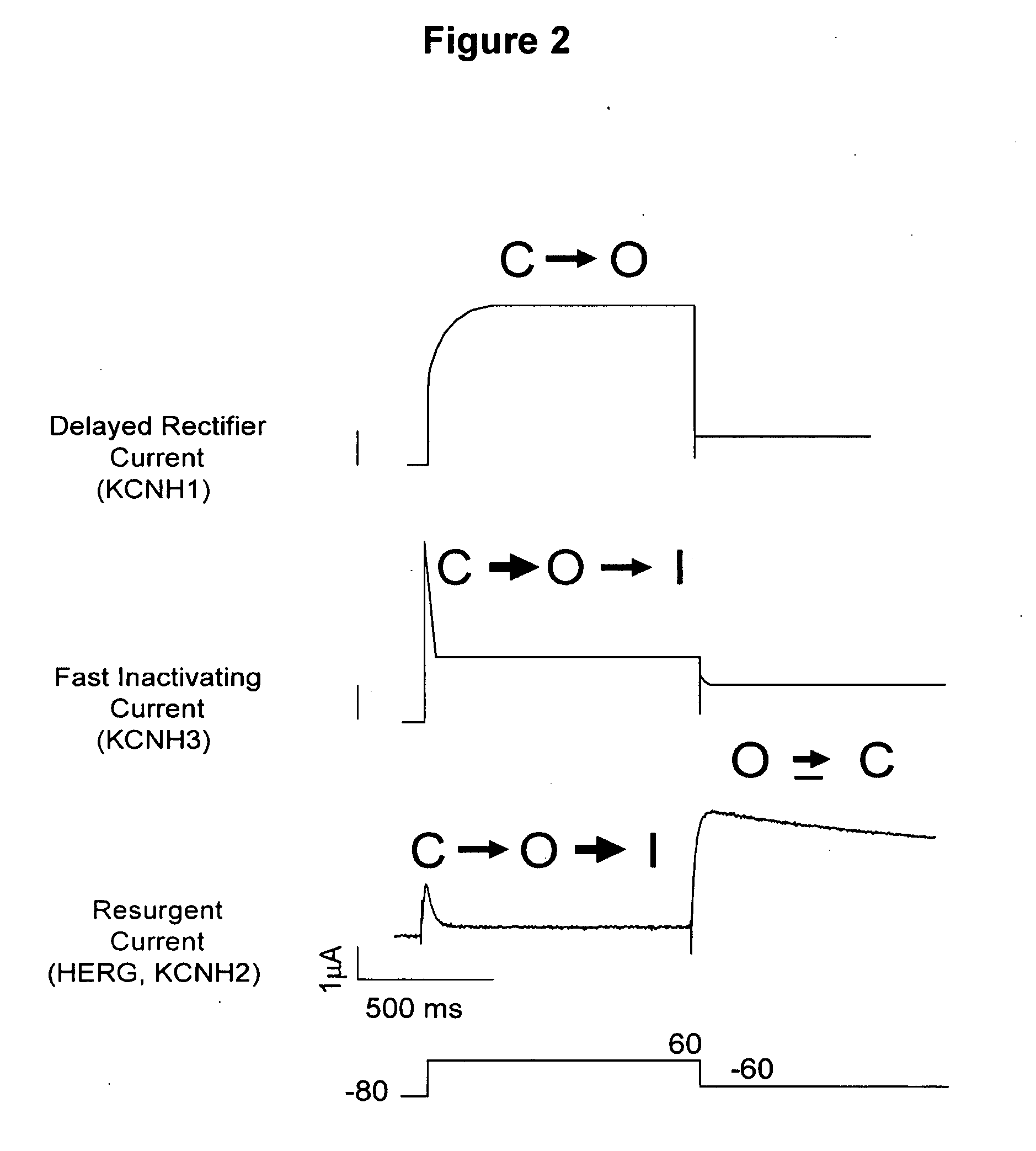
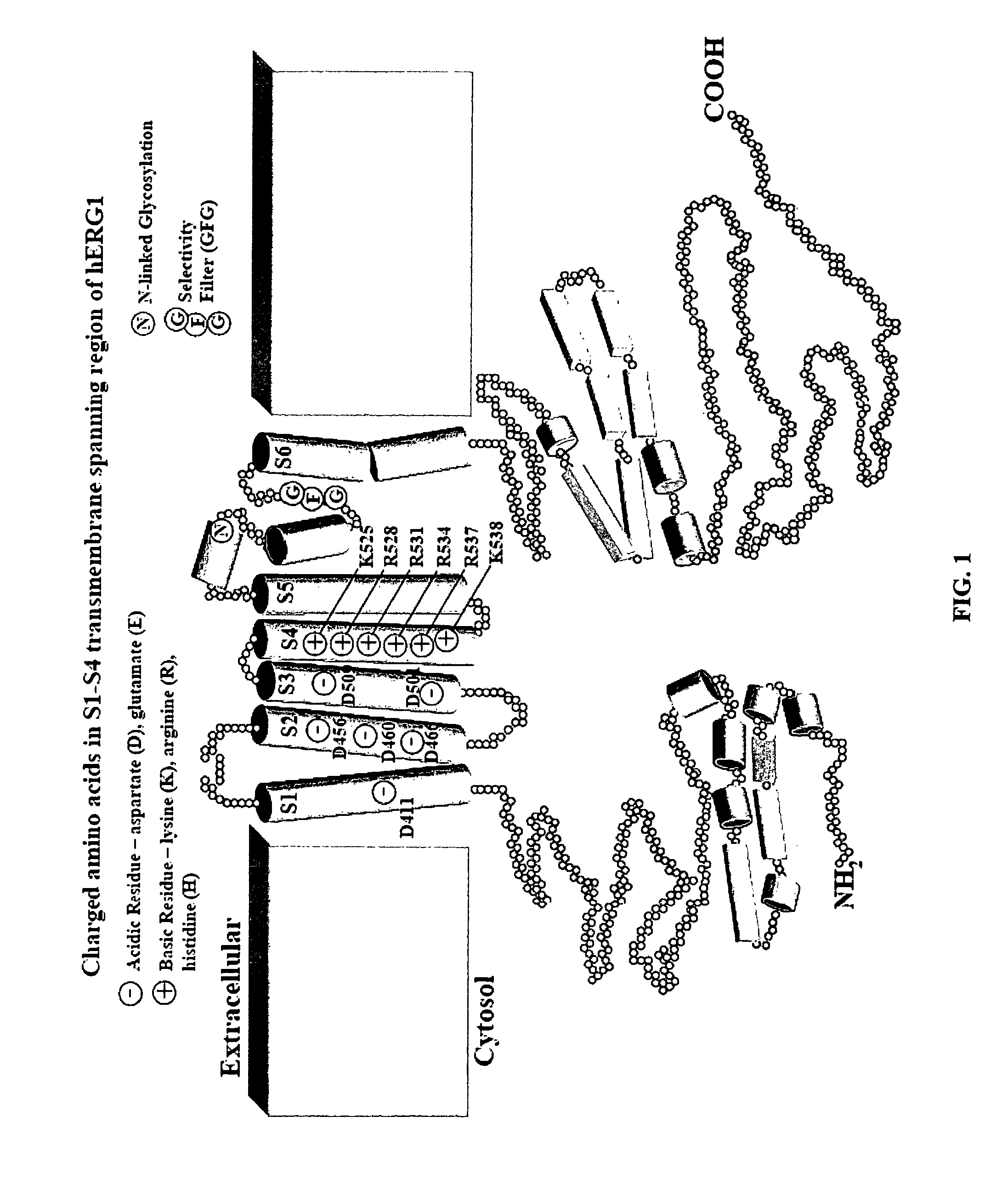
HERG (the human Ether-à-go-go-Related Gene) is a gene (KCNH2) that codes for a protein known as Kv11.1, the alpha subunit of a potassium ion channel.
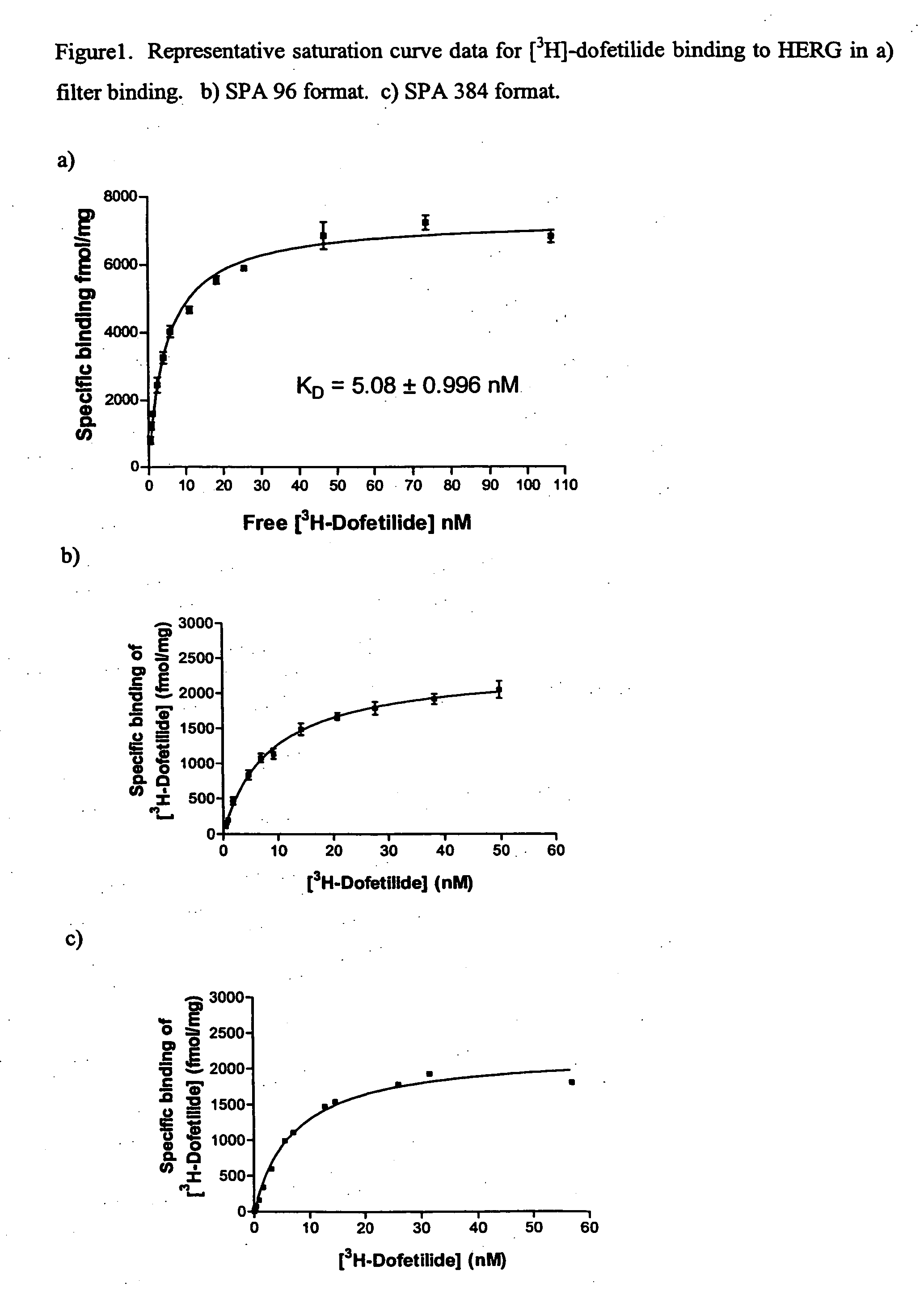
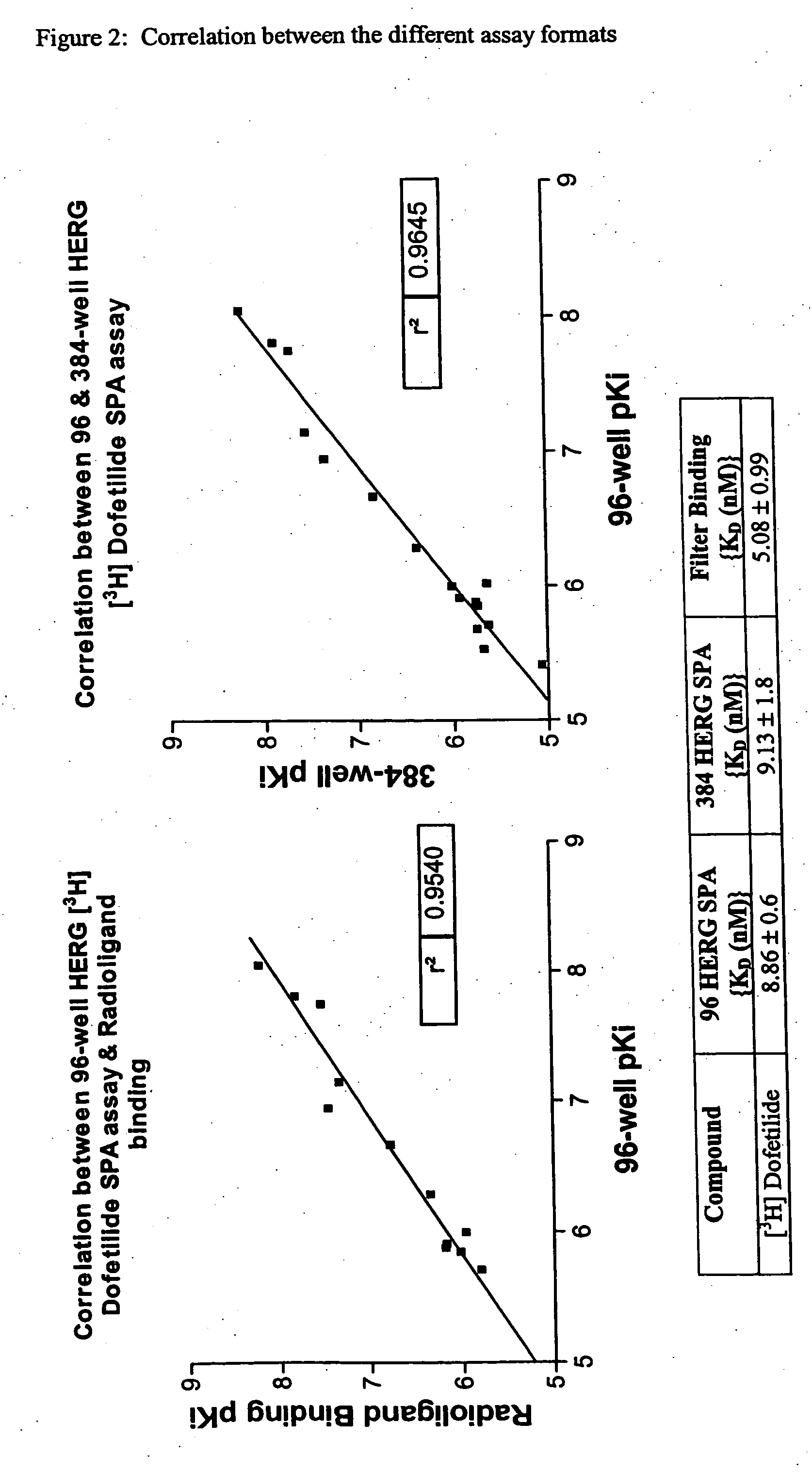
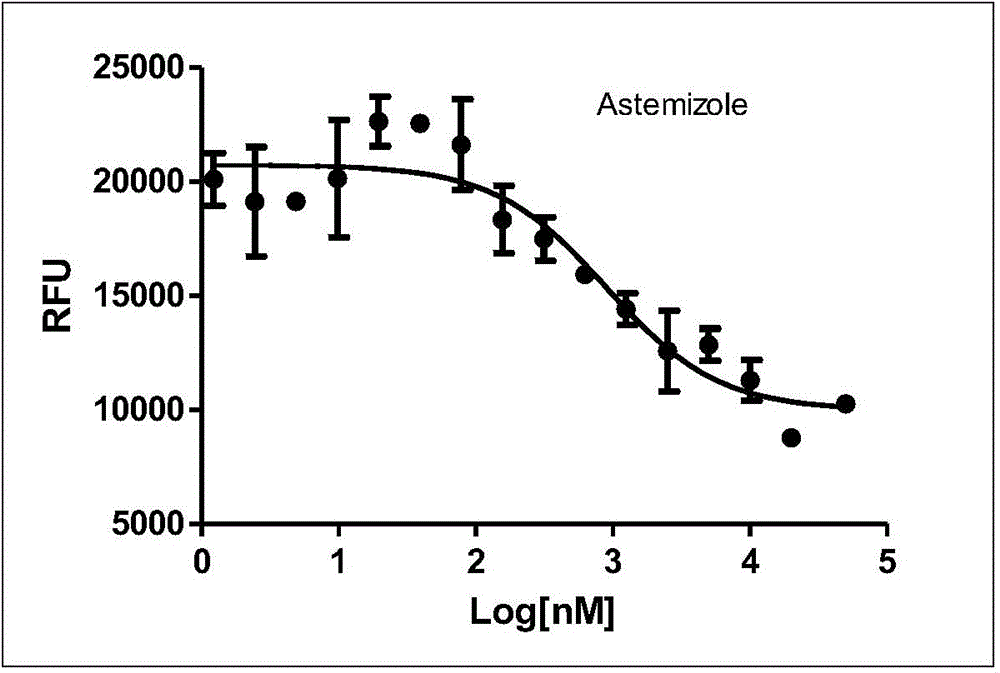
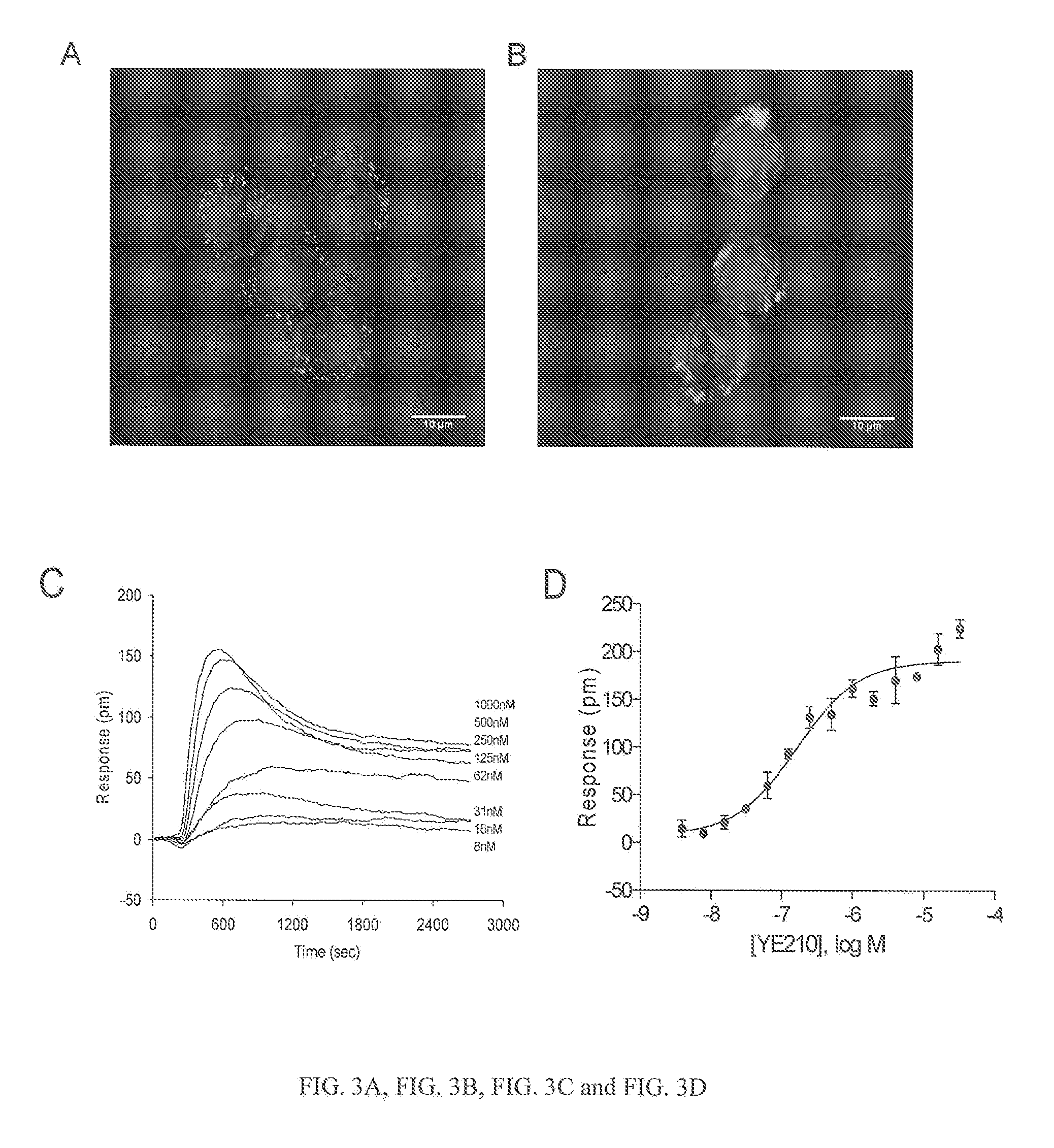
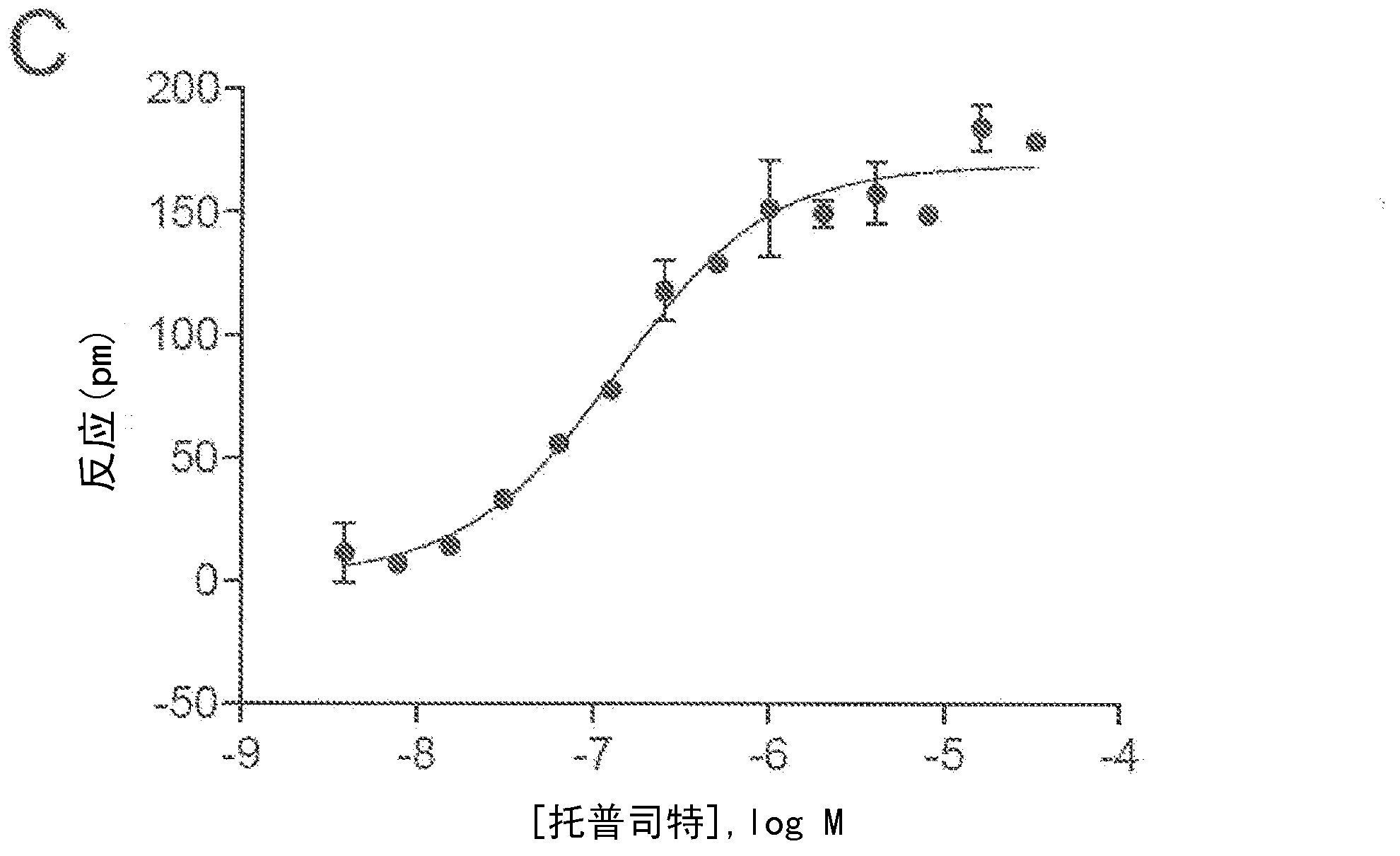
FLUORESCENCE POLARIZATION hERG ASSAY
ActiveUS20090253148A1Easy to operateAvoid issuingOrganic chemistryArtificial cell constructsHUMAN ETHER-A-GO-GO-RELATED GENECardiotoxicity
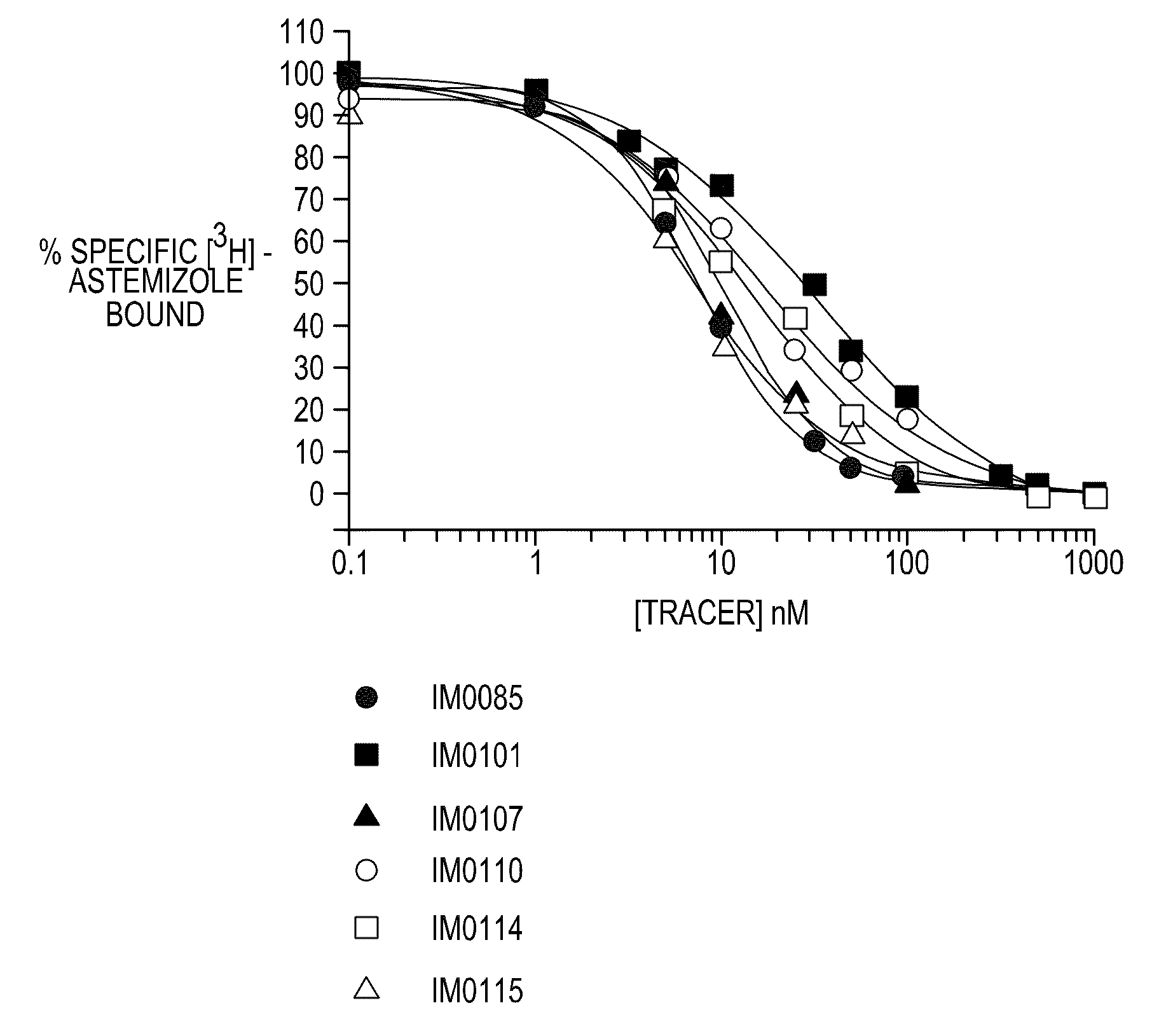
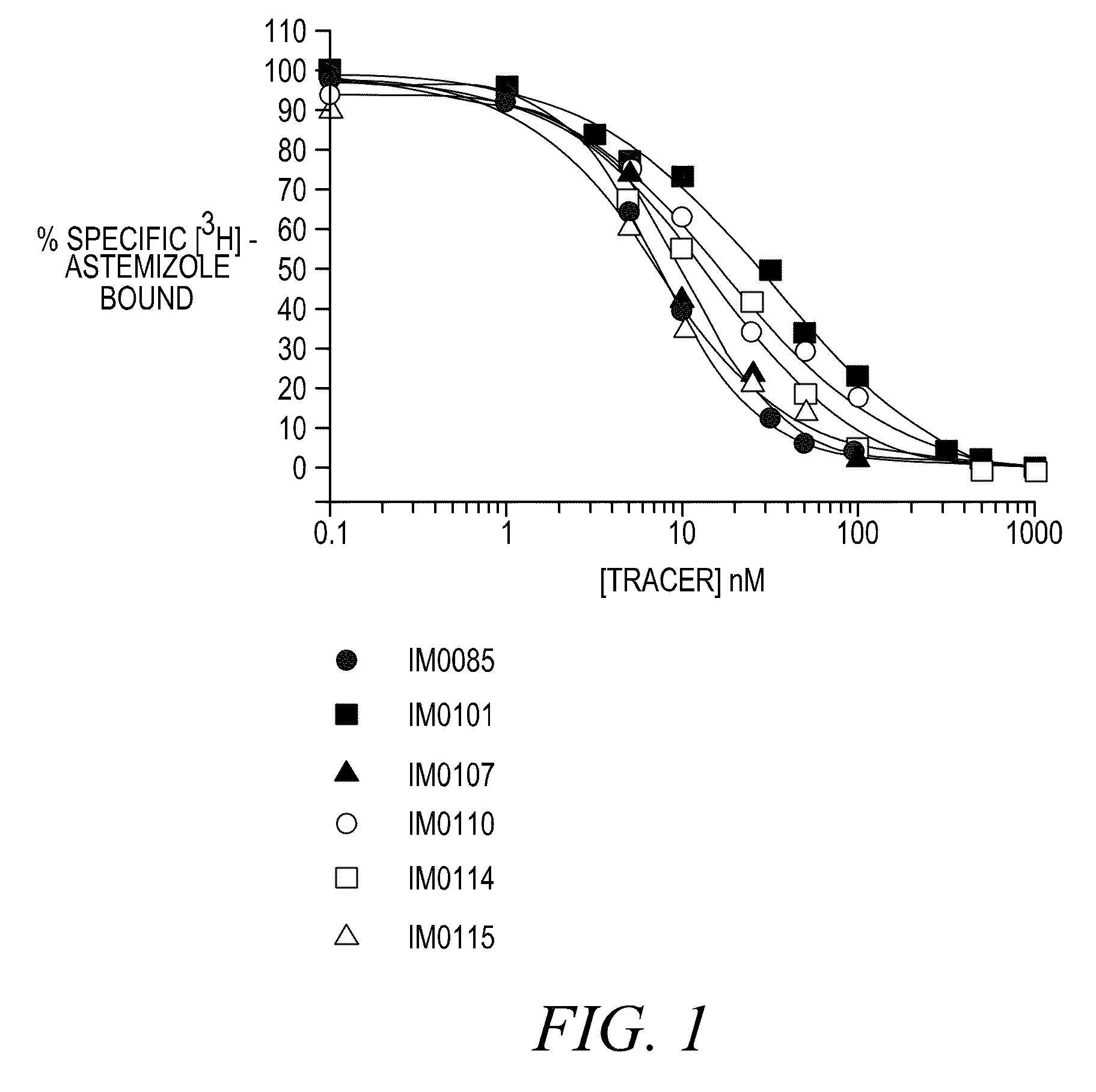
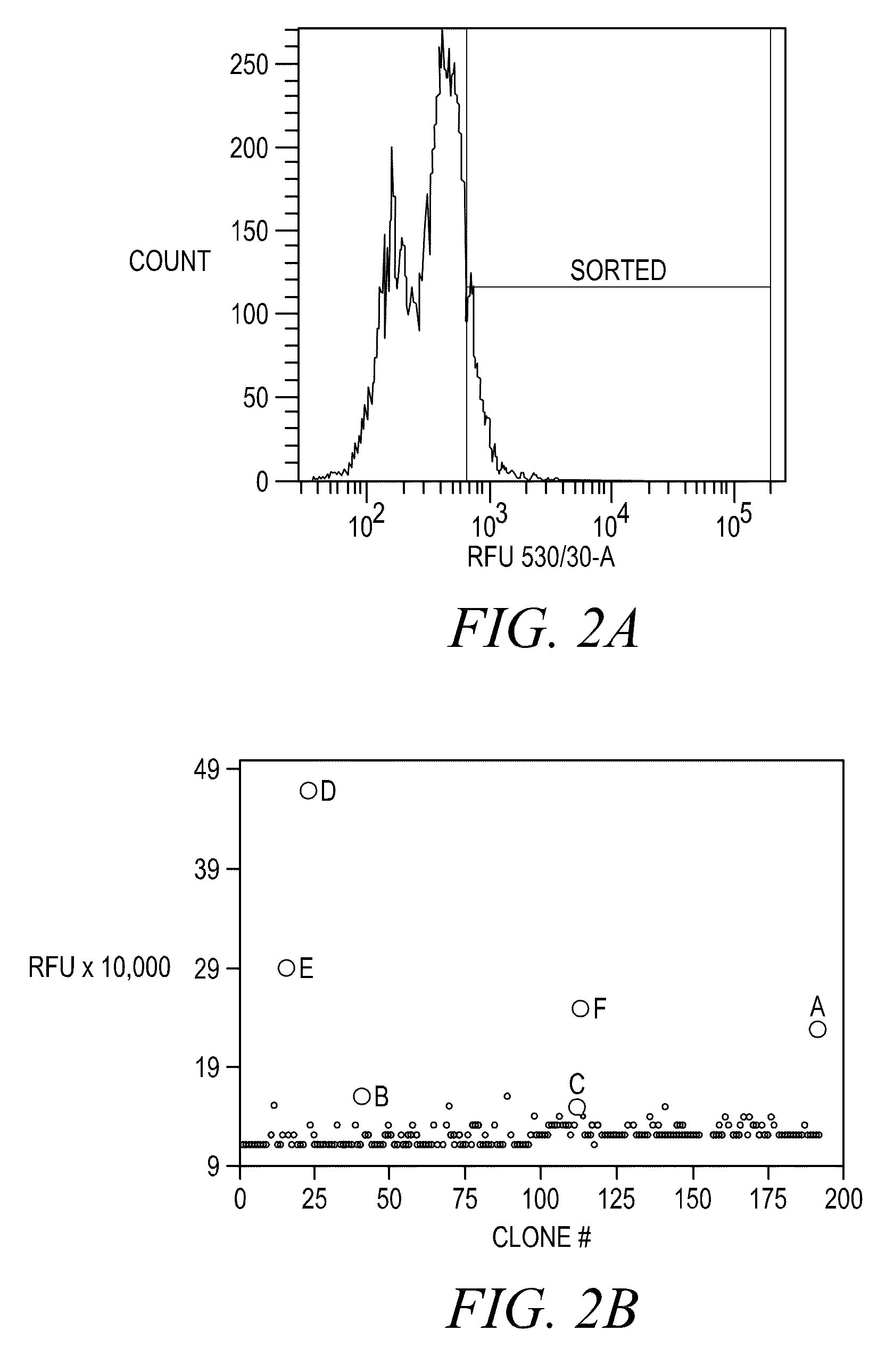
Disclosed are assays, methods, and kits for the screening of test compounds for their capability to induce cardiotoxicity in a subject. In particular, whether a test compound has the effect to prolong the Q-T interval as measured by an electrocardiogram in a human. The assays, methods, and kits disclosed herein make use of the binding interaction between novel fluorescent tracers and the hERG K+ channel, and the propensity of a test compound to influence that binding interaction.
Owner:LIFE TECH CORP
Assay
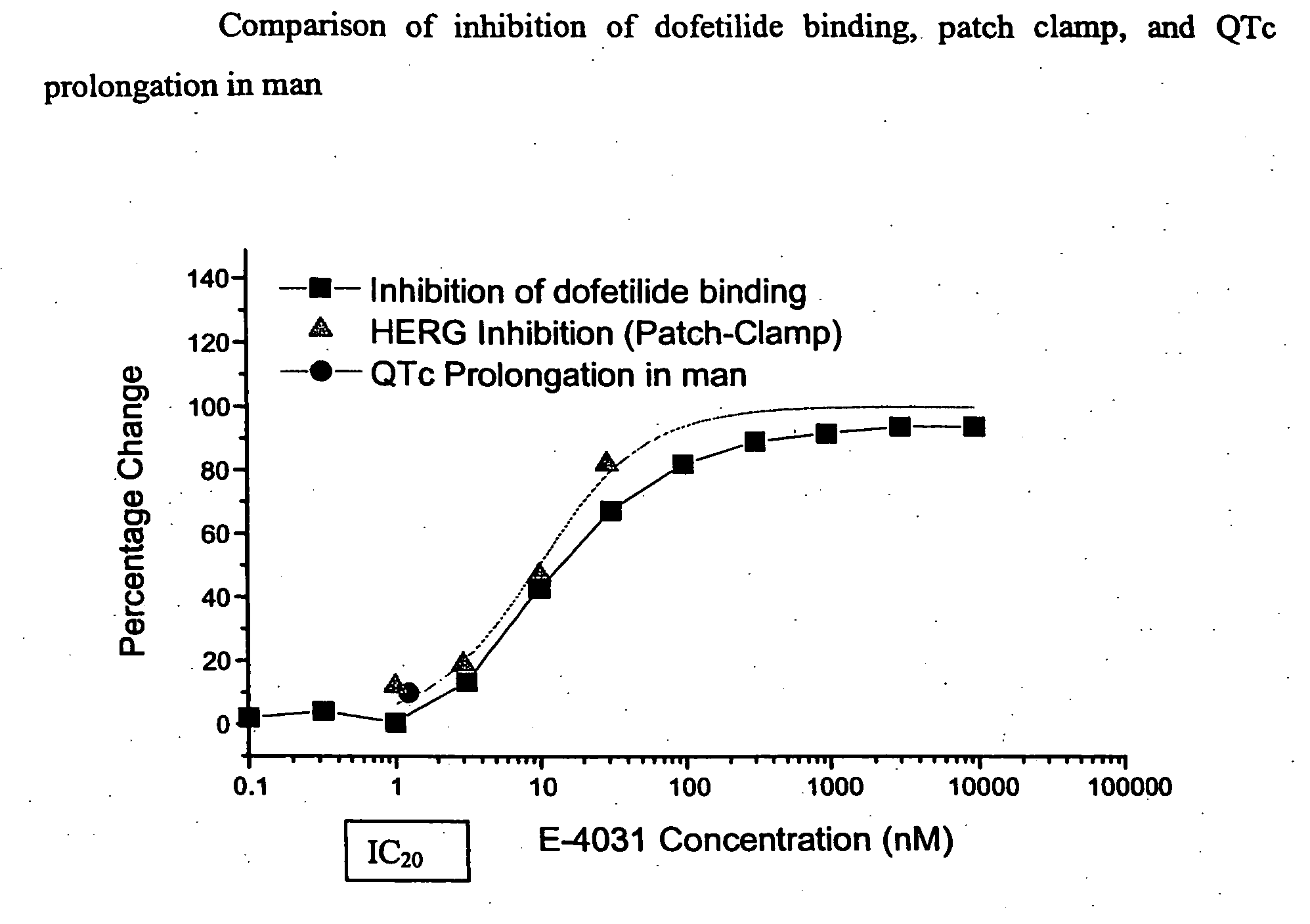
The present invention relates to assays useful for predicting whether a compound has the effect to prolong the QT interval as measured by an electrocardiogram in a human. These assays make use of the binding action of dofetilide to the HERG K+ channel, and the propensity of a test compound to influence that binding action.
Owner:PFIZER INC
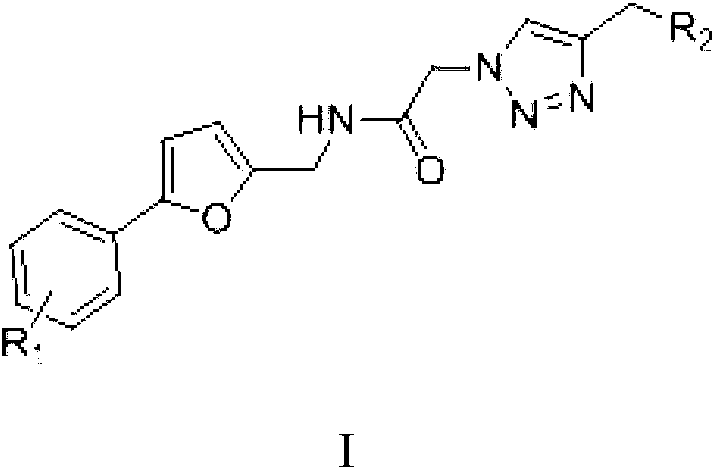
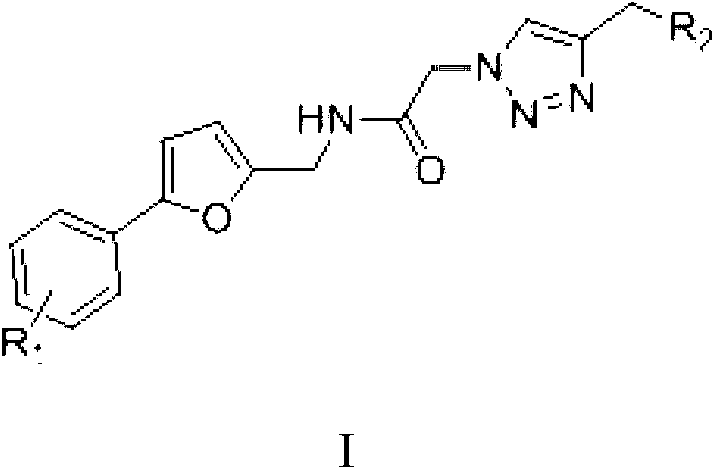
Micro-molecule fluorescent probe of phenyl furan hERG potassium channel and application thereof
ActiveCN104449670AHigh selectivityHigh sensitivityOrganic chemistryMethine/polymethine dyesFuranPotassium
The invention discloses a micro-molecule fluorescent probe of a phenyl furan hERG potassium channel and application thereof. The structure general formula of the fluorescent probe is as shown in a formula (I), wherein R1 is a single substituted group or a polysubstituted group of halogen, alkyl or alkoxy; R2 is fluorophore; n is 1-6; the piperazine ring and the fluorophore are connected by an alkyl chaing containing 1-6 carbons. The molecule of the fluorescent probe can be used for marking an hERG potassium channel, can be used for screening activity of an hERG potassium channel inhibitor and evaluating the cardiotoxicity of commercial medicines, or used as a tool drug for pharmacological, pathological and physiological researches related to hERG potassium channels. Furthermore, the preparation method of the compound is mild in reacting condition, the raw materials have low price and are easily available, and the operation and posttreatment are simple.
Owner:SHANDONG UNIV
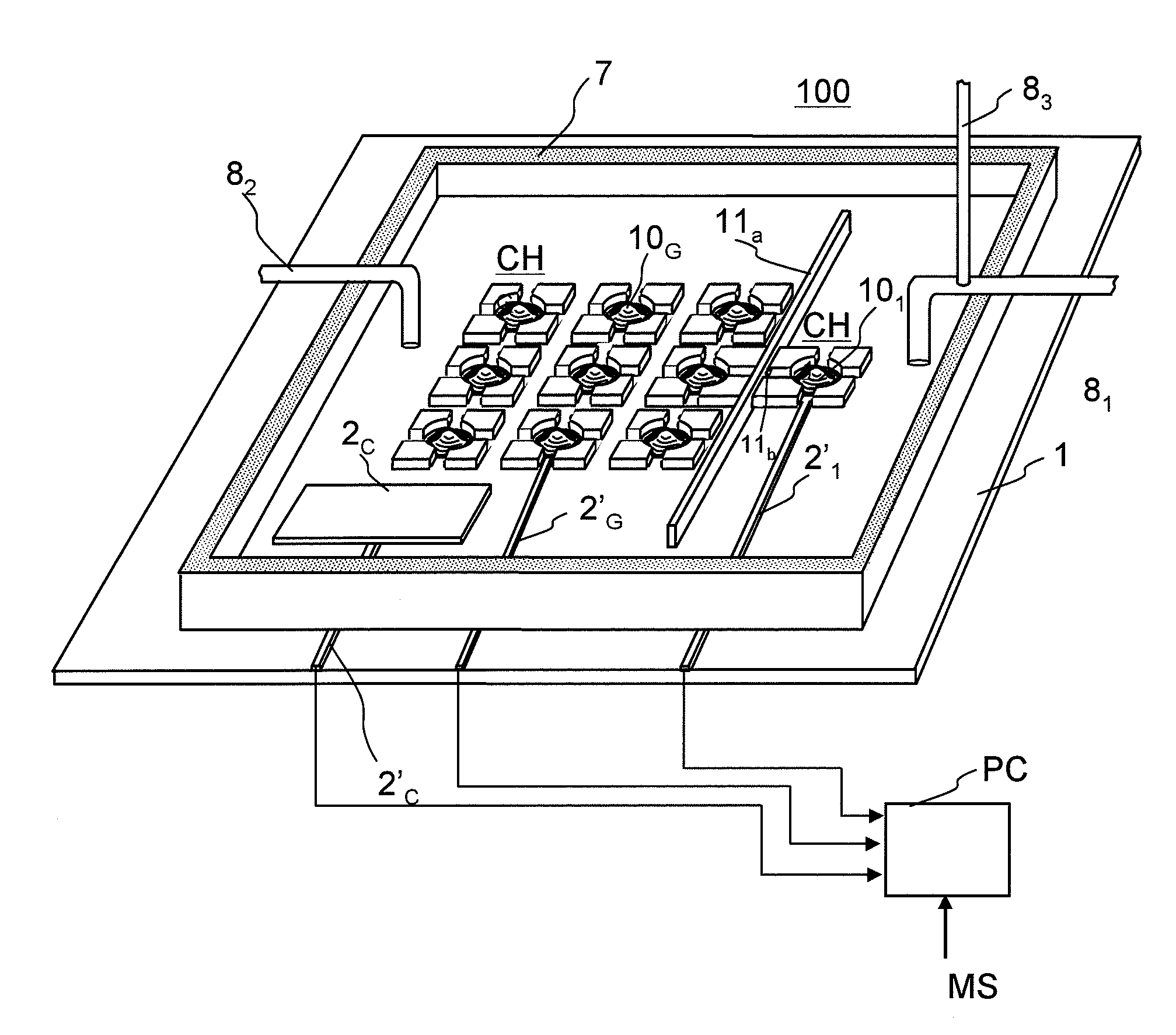
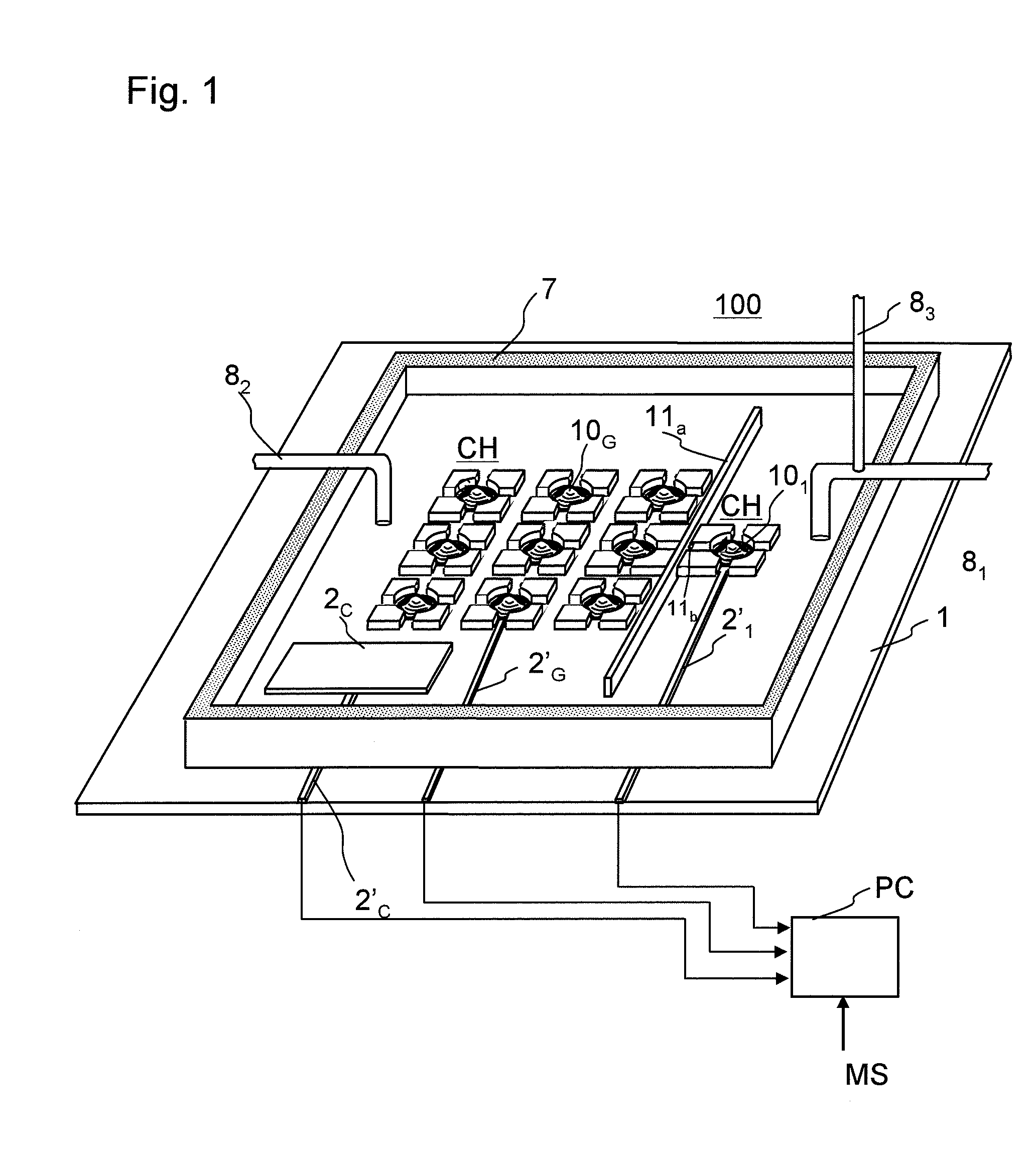
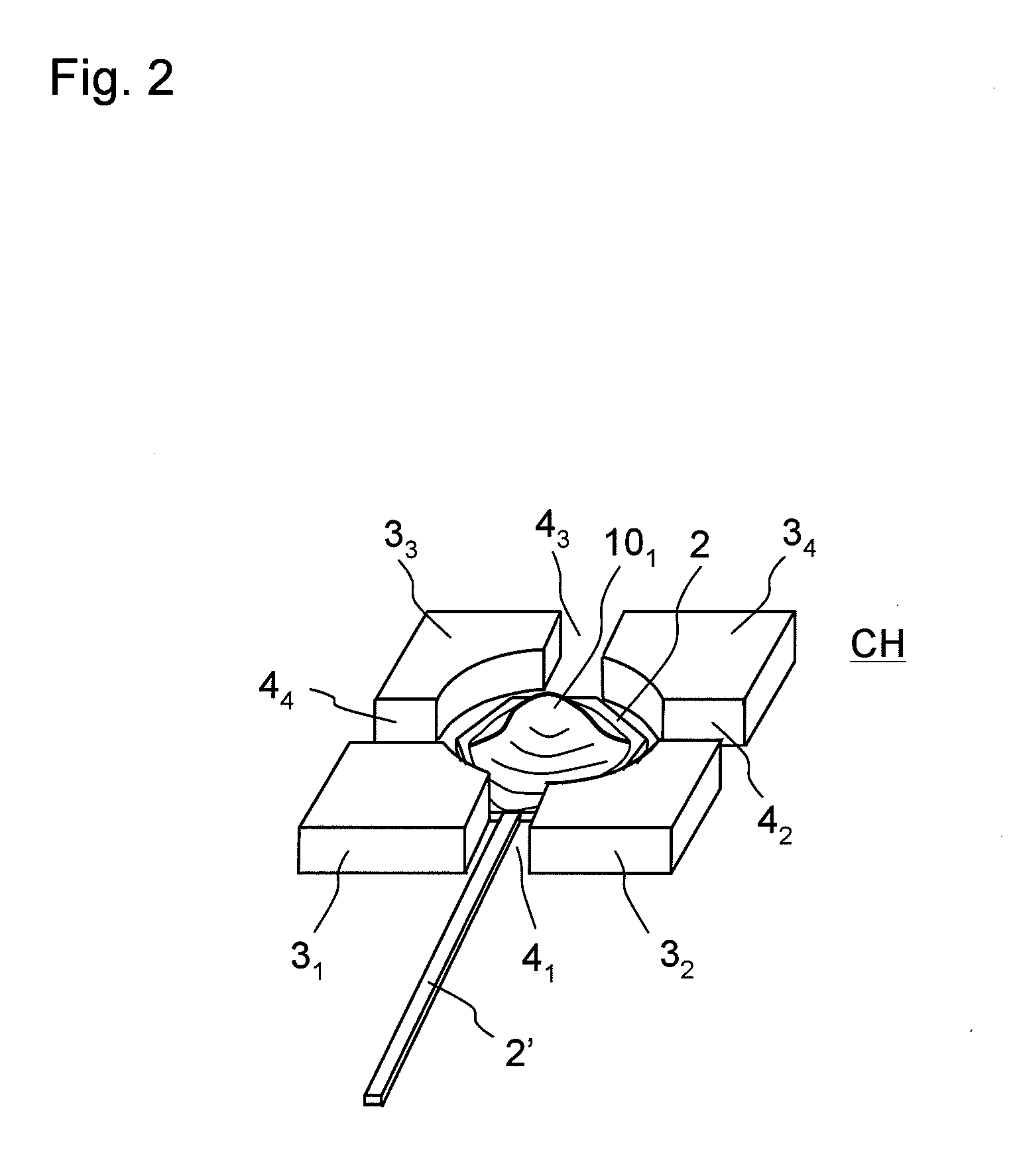
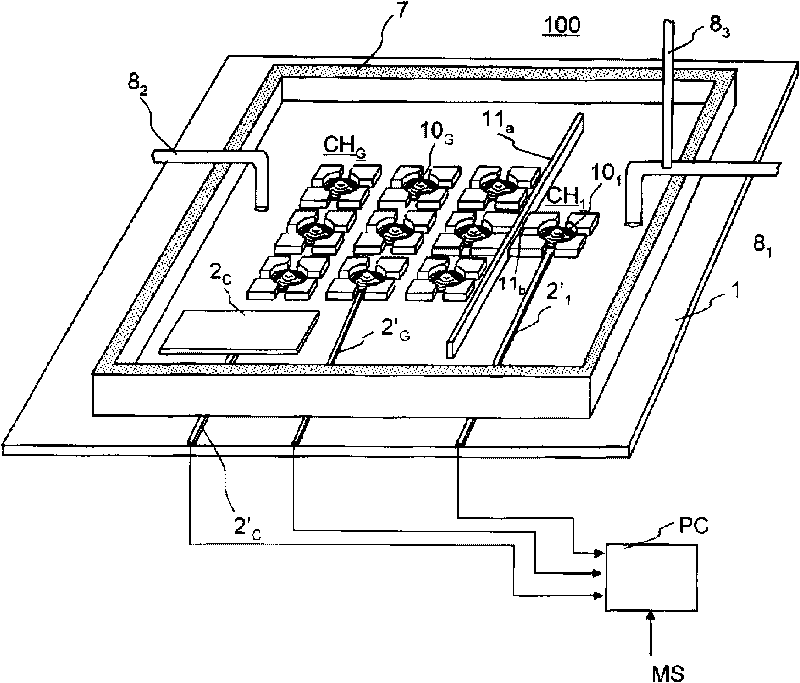
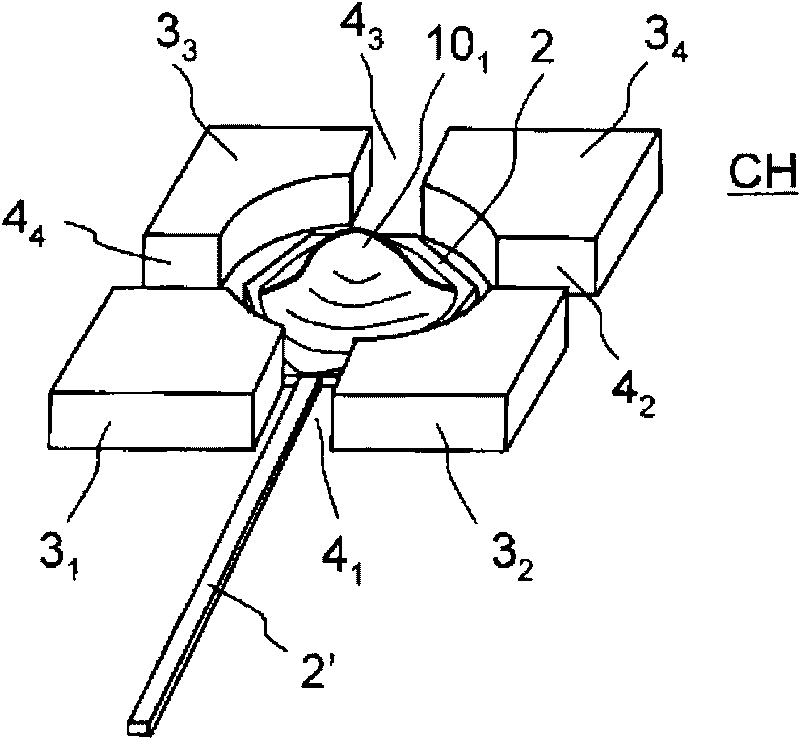
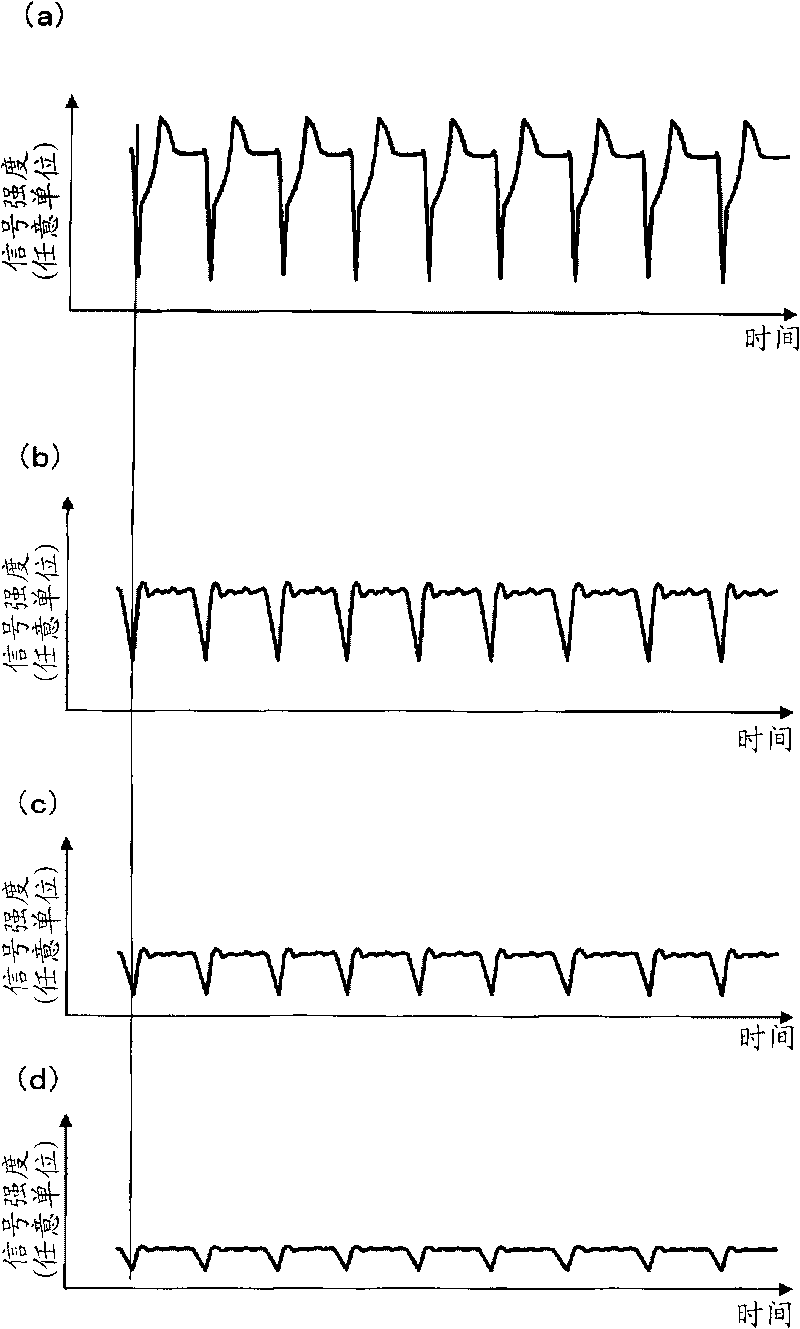
Model cell chip, apparatus for evaluating drug effect using the model cell chip and method of evaluating drug effect
InactiveUS20100178692A1Bioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoHUMAN ETHER-A-GO-GO-RELATED GENE
The present invention provides an apparatus for evaluating a drug effect enabling on-chip evaluation of the effect of a drug while the drug is acting on hERG-expressing cells. The present invention also provides a myocardial toxicity test apparatus and method therefor enabling in vitro myocardial toxicity testing that has previously been performed in vivo.A pulsating cell population and hERG-expressing cells (target model cells) are suitably isolated and arranged on a transparent substrate so that the two form gap junctions. The hERG-expressing cells are arranged on transparent electrodes provided on the transparent substrate. The hERG-expressing cells are exposed to a flow of a liquid containing a drug such that the drug acts thereon. The difference between the normal pulsation of hERG-expressing cells and the pulsation when a drug is acting thereon is captured via electric signals obtained from electrodes, and the properties of the change in potential are evaluated.
Owner:YASUDA KENJI
Acetobenzylamide piperazine derivative and application thereof as cranial nerve protective agent
ActiveCN105418506ANo risk of cardiotoxicityLittle side effectsOrganic active ingredientsNervous disorderHUMAN ETHER-A-GO-GO-RELATED GENESide effect
The invention discloses an acetobenzylamide piperazine derivative and an application thereof as a cranial nerve protective agent. Pharmacological experiments verify that the compound disclosed by the invention shows an obvious effect against glutamic acid-induced neuron exitotoxicity in vitro and obvious anti-anoxia activity in a mouse, and a herg test further shows that the compound disclosed by the invention does not have the risk of cardiotoxicity. Therefore, the compound disclosed by the invention has the advantages of being high in activity, less in side effect and good in druggability. The compound and a medicinal preparation thereof have a good curative effect for treating cranial nerve injury diseases, for example, stroke and related diseases and do not have the risk of cardiotoxicity. The acetobenzylamide piperazine derivative is a free alkali or salt of a compound with a chemical structural formula shown in the description.
Owner:SHANGHAI INST OF PHARMA IND +1
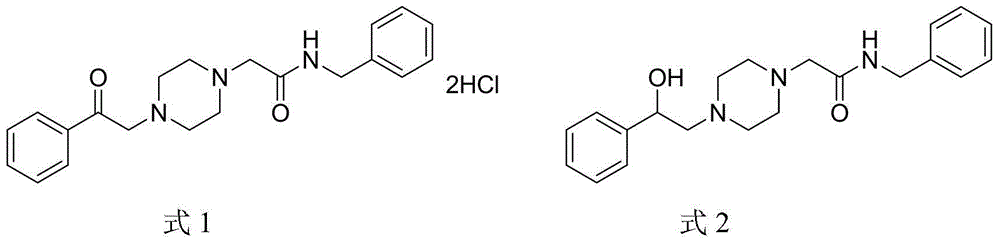
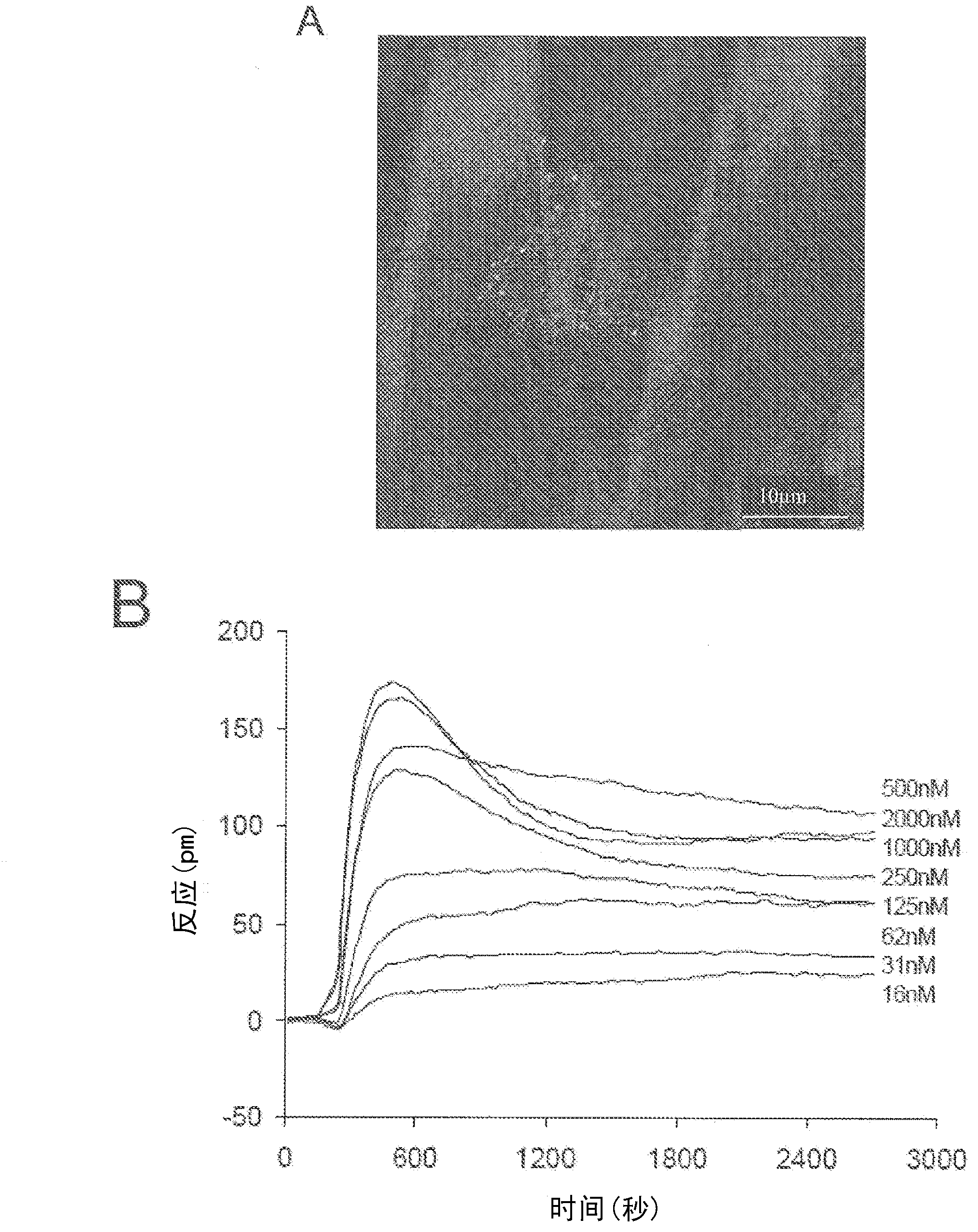
Compositions and methods for the treatment of pathological condition(s) related to GPR35 and/or GPR35-herg complex
InactiveUS20120022116A1Prevent and treat diseaseDesired sizeBiocideOrganic chemistryPathophysiologyHUMAN ETHER-A-GO-GO-RELATED GENE
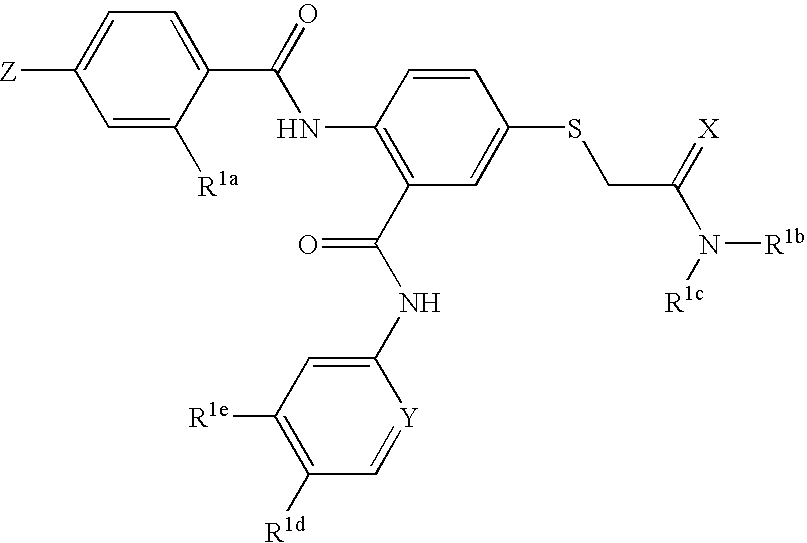
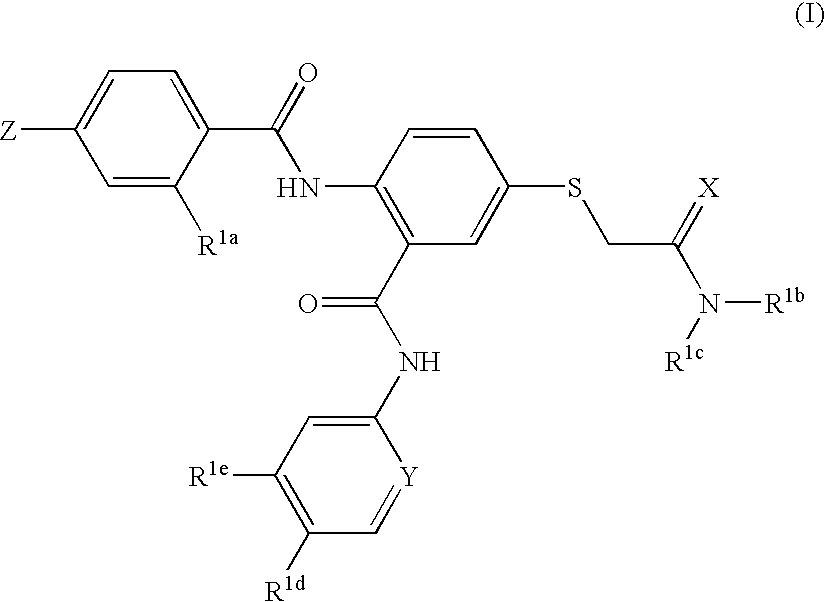
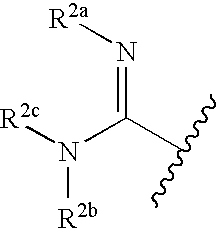
Disclosed are compositions and methods for the prevention and / or treatment of diseases which are pathophysiologically related to GPR35, and / or GPR35-hERG signaling complex. For example, disclosed are compounds for preventing and / or treating diseases which are pathophysiologically related to GPR35 in a subject. The compounds having a formula (I), (II) or (III):
Owner:CORNING INC
Natural medicinal addictive for napkin and its preparation method
The invention concerns a natural healthy drug addictive for napkin and the preparation process thereof. The components in weight portion are as follows: cyperirhizome 5-20, Chinese mugwort leaf 5-20, Chinese angelica root 5-20, corydalis tuber 5-20, cassia twig 1-9, asarum herg 1-5. It adopts supercritical CO#-[2] fluid extracting method to extract effective components of the Chinese herbal medicine, then regulates the distilled water decoction acidity of dark plum, the flavor and property are mild and having no stimuli to skin. The drug addictive has functions in activating blood circulation, dissipating blood stasis, warming the channel to relieve pain. It can relieve dysmenorrhea caused by liver qi stagnation and blood circulation obstruction. The invention has certain actions in preventing and treating all kinds of colpitis, pelvic inflammatory disease and urethritis.
Owner:XIAMEN UNIV
Benzofuran compound, preparation method thereof and application of benzofuran compound in preparation of antiarrhythmic drugs
ActiveCN103012381AStrong antiarrhythmic activityNovel structureOrganic active ingredientsOrganic chemistrySodium ascorbateCycloaddition
The invention relates to a benzofuran compound, a preparation method thereof and an application of the benzofuran compound in preparation of antiarrhythmic drugs. The structural formula of the compound and substituent groups of the compound are described in the specification; and the preparation method comprises the following steps of: reacting R1-substituted phenylamine with sodium nitrite under an acidic condition firstly to generate diazonium salt, then carrying out coupled reaction with furfuryl amine in presence of copper chloride or titanium trichloride to generate an intermediate III, stirring the intermediate III and chloroacetyl chloride in K2CO3 and CH2Cl2 at room temperature and reacting to obtain an intermediate IV, carrying out refluxing of the intermediate IV and sodium azide in acetonitrile and stirring and reacting to obtain an intermediate V; and carrying out cycloaddition reaction on the intermediate V and a compound VII under the catalysis of sodium ascorbate and CuSO4 to obtain the target compound. The compound provided by the invention has the activity of inhibiting an hERG potassium ion channel and can be utilized as a leading compound of the antiarrhythmic drugs.
Owner:SHANDONG UNIV
METHOD FOR ESTIMATING hERG INHIBITION OF DRUG CANDIDATES USING MULTIVARIATE PROPERTY AND PHARMACOPHORE SAR
InactiveUS20080227100A1Inhibitory activityRaise the possibilityChemical property predictionMolecular designMedicinePharmacophore
The present invention provides a computational model and methods of use thereof for predicting whether a compound is likely to inhibit K+ flow through the hERG ion channel. Methods for in silico screening of compounds that have a lower likelihood of inhibiting hERG are also provided.
Owner:BRISTOL MYERS SQUIBB CO
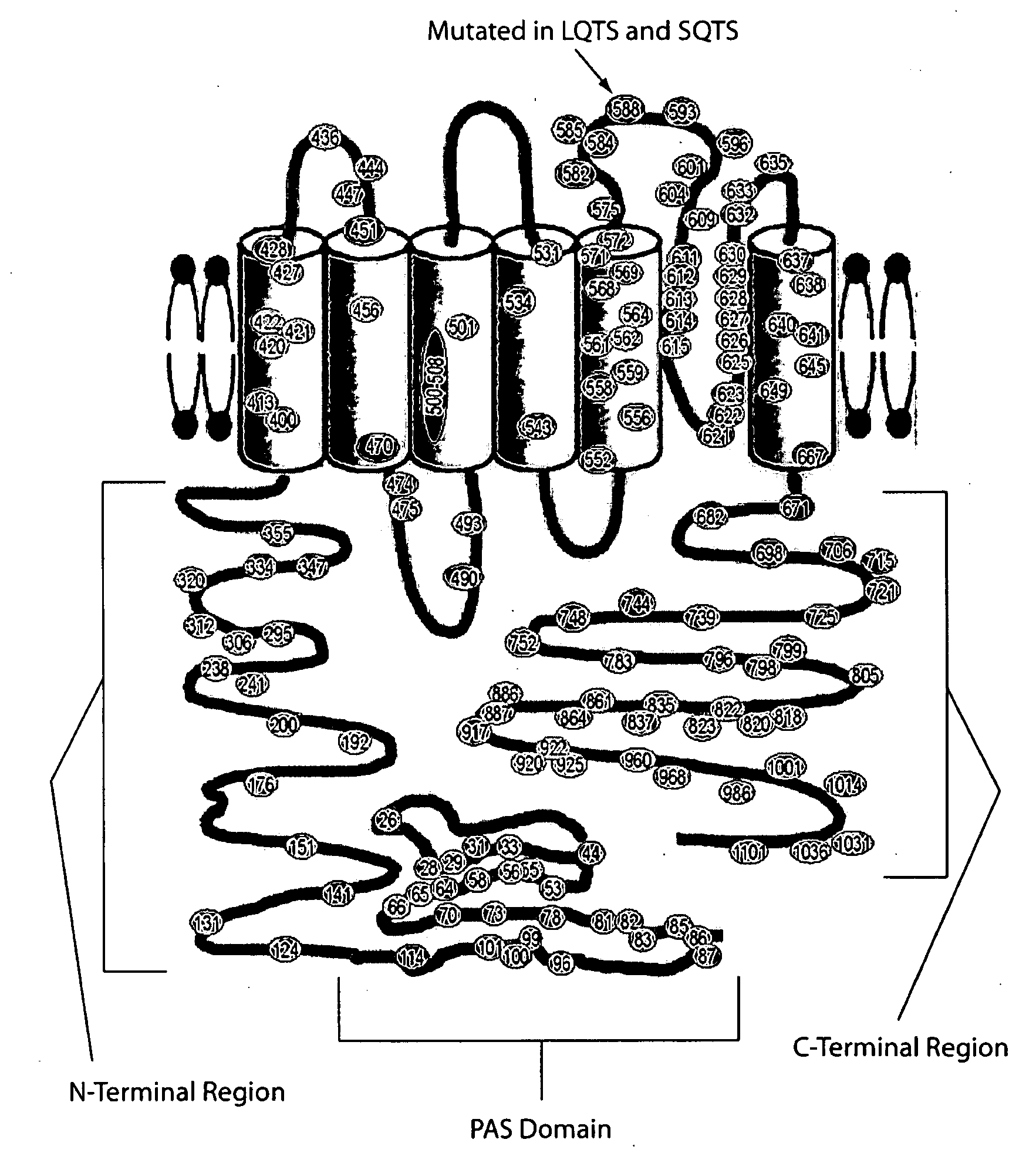
Mutations Associated with the Long QT Syndrome and Diagnostic Use Thereof
The present invention is based on the identification of new mutations in KCNQ1 (also termed KvLQTI), KCNH2 (also termed HERG), SCN5A, KCNE1 (also termed minK), KCNE2 (also termed MiRP) genes that encode ionic channels involved in cardiac electrical activity and are potentially responsible for the Long QT Syndrome. According to a main aspect, the invention relates to nucleic acids, oligonucleotides and polynucleotides and mRNA, containing sequences of KCNQ1, KCNH2 SCN5A, KCNE1, KCNE2 genes and cDNAs in a mutated form and to respective variant proteins thereof. A preferred embodiment of the present invention is represented by a diagnostic method based on the identification of a group of about 70 non-private mutations in the KCNQ1, KCNH2 and SCN5A genes, detected at high frequency. The method, which is able to identify about 40% of the probands, is non exclusively based on identification of mutations that are described and characterized in this invention where said identification has both prognostic and diagnostic value for the Long QT Syndrome.
Owner:FOND SALVATORE MAUGERI CLINICA DEL LAVORO E DELLA RIABILITAZIONE +1
Salts prepared from 2-(1-acyloxy-n-amyl)benzoic acid and basic amino acid or aminoguanidine, and preparation method and application thereof
ActiveCN109678715AImprove performanceGood water solubilityOrganic active ingredientsOrganic compound preparationSolubilityAcute toxicity testing
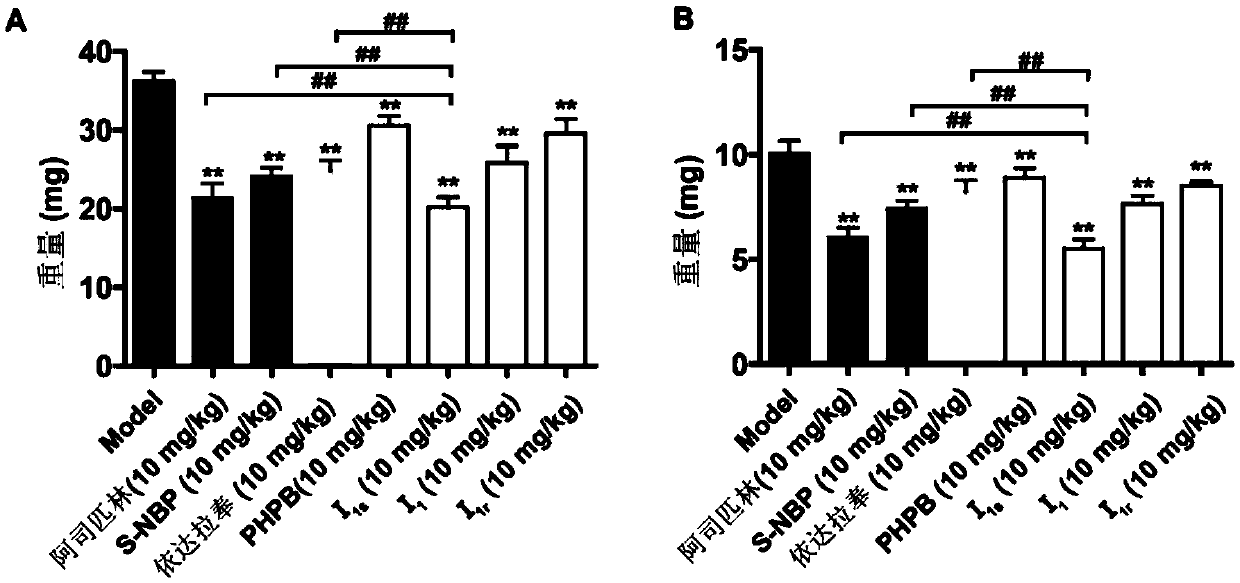
The invention discloses salts prepared from 2-(1-acyloxy-n-amyl)benzoic acid and basic amino acid or aminoguanidine, a preparation method thereof, a medicinal preparation containing the salts, and application of the salts to preparing of medicines used for preventing or treating ischemic cardiovascular and cerebral vascular diseases, resisting thrombus and relieving cardio-cerebral circulatory disturbance. A compound not only has good water solubility, aqueous solution stability and pharmacokinetics performance, but also has strong activity for resisting platelet aggregation, resisting thrombus, resisting cerebral ischemia and protecting nerves, the effect is superior to that of (S)-butylphthalide and (R / S)-2-(1-hydroxy-n-amyl)potassium benzoate salt (PHPB), acute toxicity of the compoundto mouse intravenous injection dosing is remarkably lower than that of butylphthalide and PHPB, the inhibition ratio of the compound to an hERG potassium channel of a CHO-hERG cell is lower than thatof the (S)-butylphthalide, and a result of a bacterial reverse mutation test (Ames test) is negative.
Owner:JIANGSU KANION PHARMA CO LTD
Model cell chip, apparatus for evaluating drug effect using the model cell chip and method of evaluating drug effect
InactiveCN101711276AMeasuring Toxicity CorrectlyBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGap Junction Proteins
The present invention provides an apparatus for evaluating a drug effect enabling on-chip evaluation of the effect of a drug while the drug is acting on hERG-expressing cells. The present invention also provides a myocardial toxicity test apparatus and method therefor enabling in vitro myocardial toxicity testing that has previously been performed in vivo. A pulsating cell population and hERG-expressing cells (target model cells) are suitably isolated and arranged on a transparent substrate so that the two form gap junctions. The hERG-expressing cells are arranged on transparent electrodes provided on the transparent substrate. The hERG-expressing cells are exposed to a flow of a liquid containing a drug such that the drug acts thereon. The difference between the normal pulsation of hERG-expressing cells and the pulsation when a drug is acting thereon is captured via electric signals obtained from electrodes, and the properties of the change in potential are evaluated.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV
ERG-1 Peptides and Polynucleotides and Their Use in the Treatment and Diagnosis of Disease
Owner:UNIV OF MARYLAND
Compositions and methods for the treatment of pathological condition(s) related to GPR35 and/or GPR35-HERG complex
InactiveCN103237791AOrganic chemistryMetabolism disorderHUMAN ETHER-A-GO-GO-RELATED GENEPathophysiology
Disclosed are compositions and methods for the prevention and / or treatment of diseases which are pathophysiologically related to GPR35, and / or GPR35-HERG signaling complex. For example, disclosed are compounds for preventing and / or treating diseases which are pathophysiologically related to GPR35 in a subject.
Owner:CORNING INC
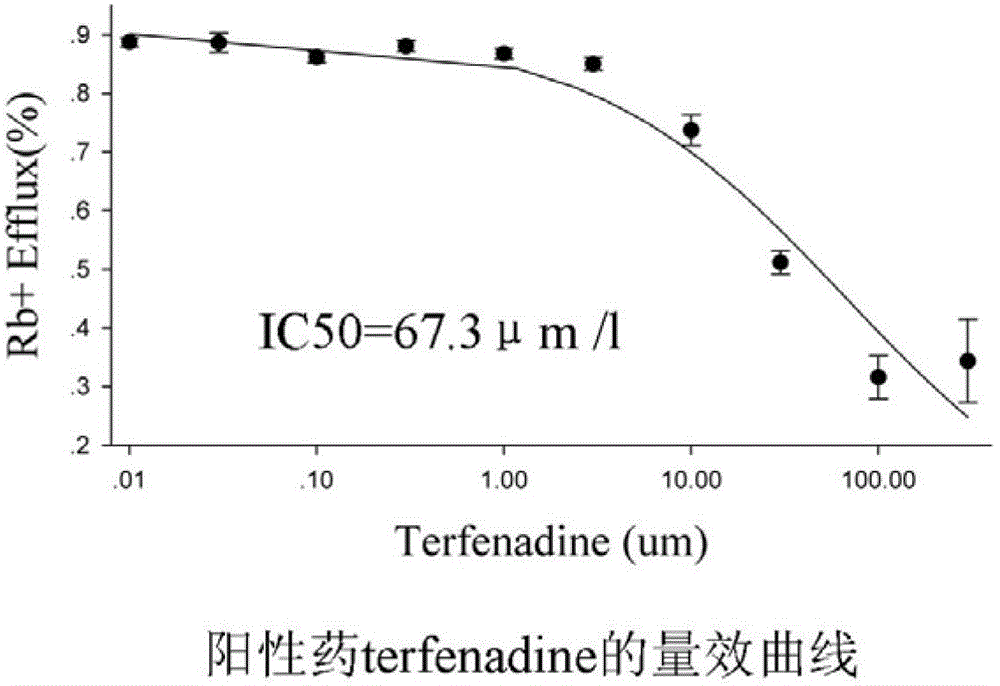
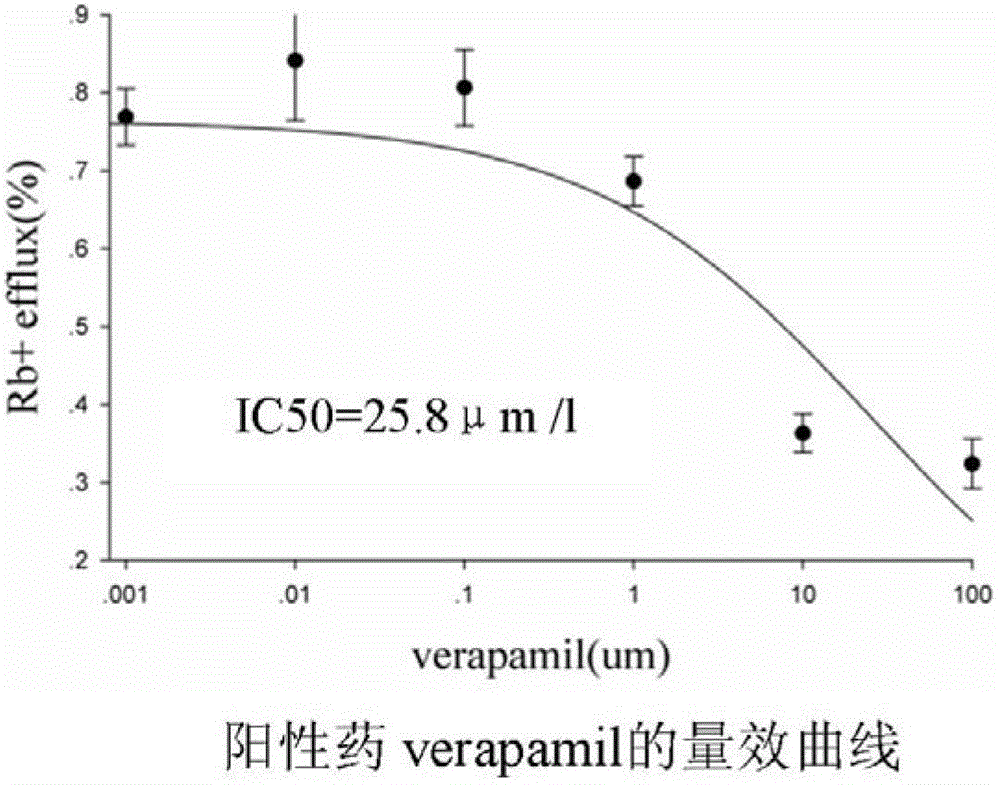
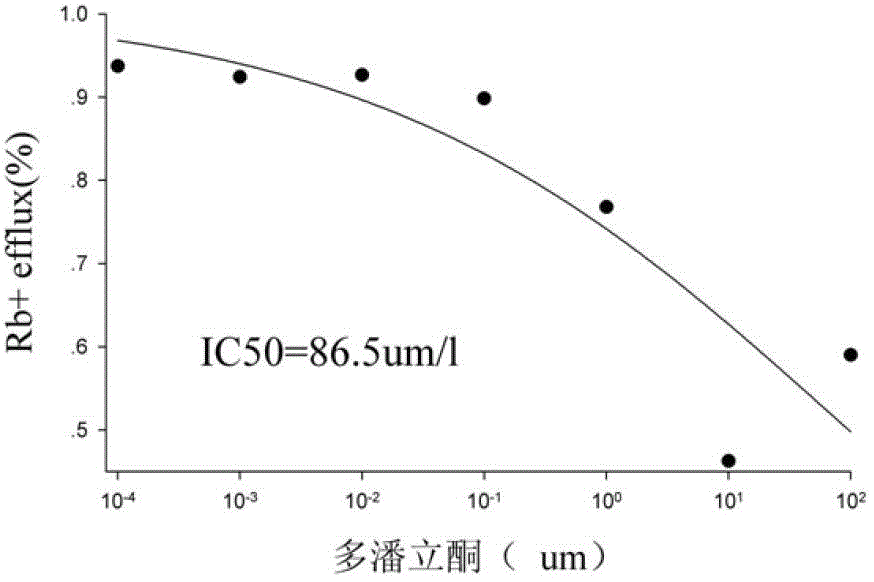
Method for efficiently evaluating safety of medicine in hERG channel
InactiveCN102747129ALower requirementSimple methodMicrobiological testing/measurementColor/spectral properties measurementsIc50 valuesCardiac muscle
The invention discloses a method for efficiently evaluating the safety of a medicine in an hERG channel, characterized by firstly conducting routine culture of a cell strain expressing the hERG channel, then removing the culture medium and adding the cell strain into a fixed buffer, incubating for 1-4h under the routine culture conditions of the cell strain, removing the fixed buffer, then adding a fixed buffer containing the medicine to be evaluated and incubating for 5-40min, and then removing the fixed buffer containing the medicine to be evaluated, washing the cell strain by using a washing buffer to remove residual Rb+, adding an ion channel opening buffer and incubating for 5-20 min, removing the cell supernatant, cracking the cell strain into a cell lysate, and respectively determining the Rb+ concentrations in the cell supernatant and cell lysate; setting different concentrations of the medicine to be evaluated in the fixed buffer containing the medicine to be evaluated to repeatedly determine, acquiring an IC50 value according to obtained data of the determination of the medicine to be evaluated with different concentrations, wherein if the IC50 value is no larger than 1000 mumol / L, the medicine to be evaluated has a certain inhibition on the hERG channel, and the lower the IC50 value is, the higher the inhibition is and the larger the risk of cardiotoxicity is.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Methods for Treating GI Tract Disorders
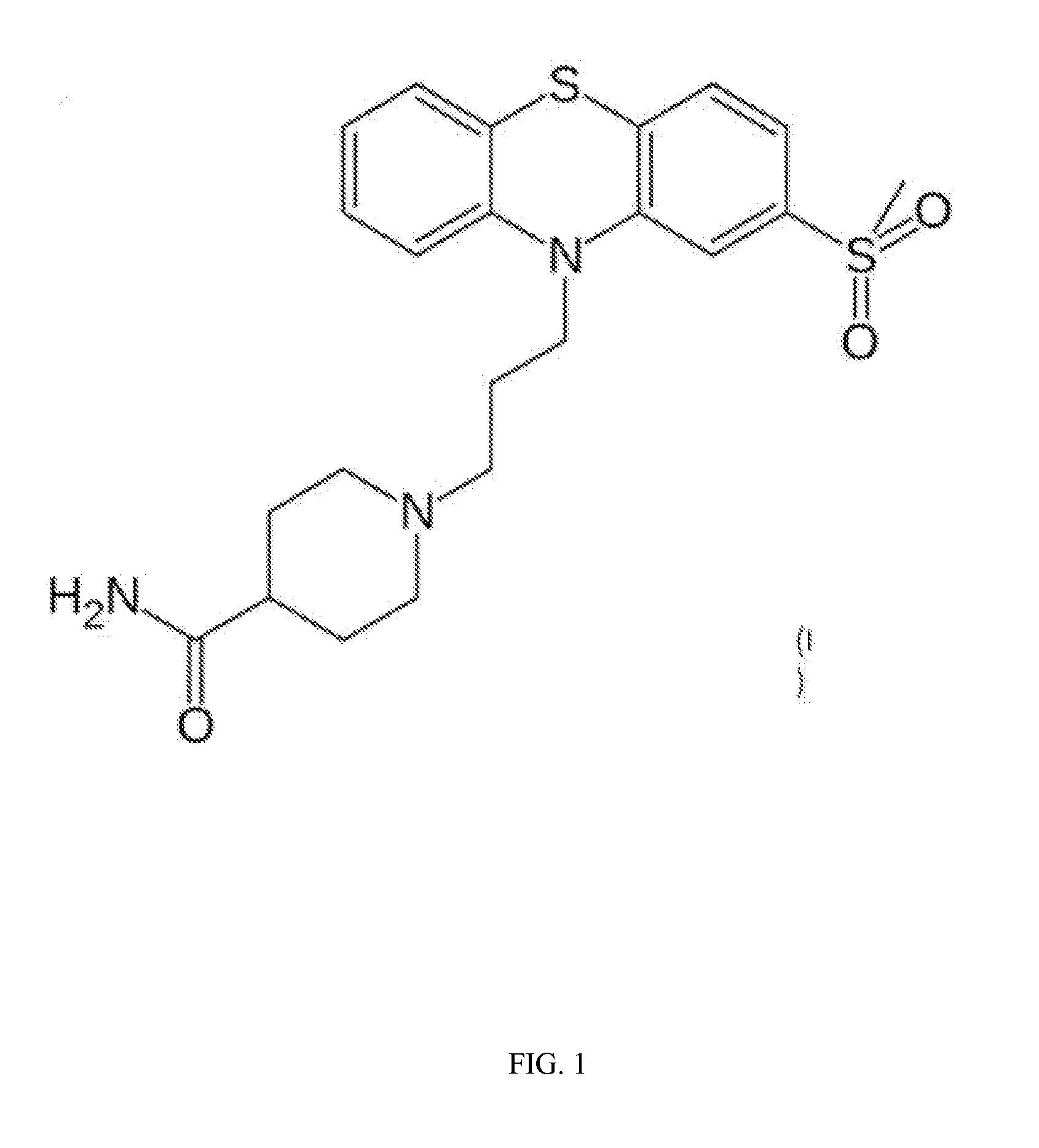
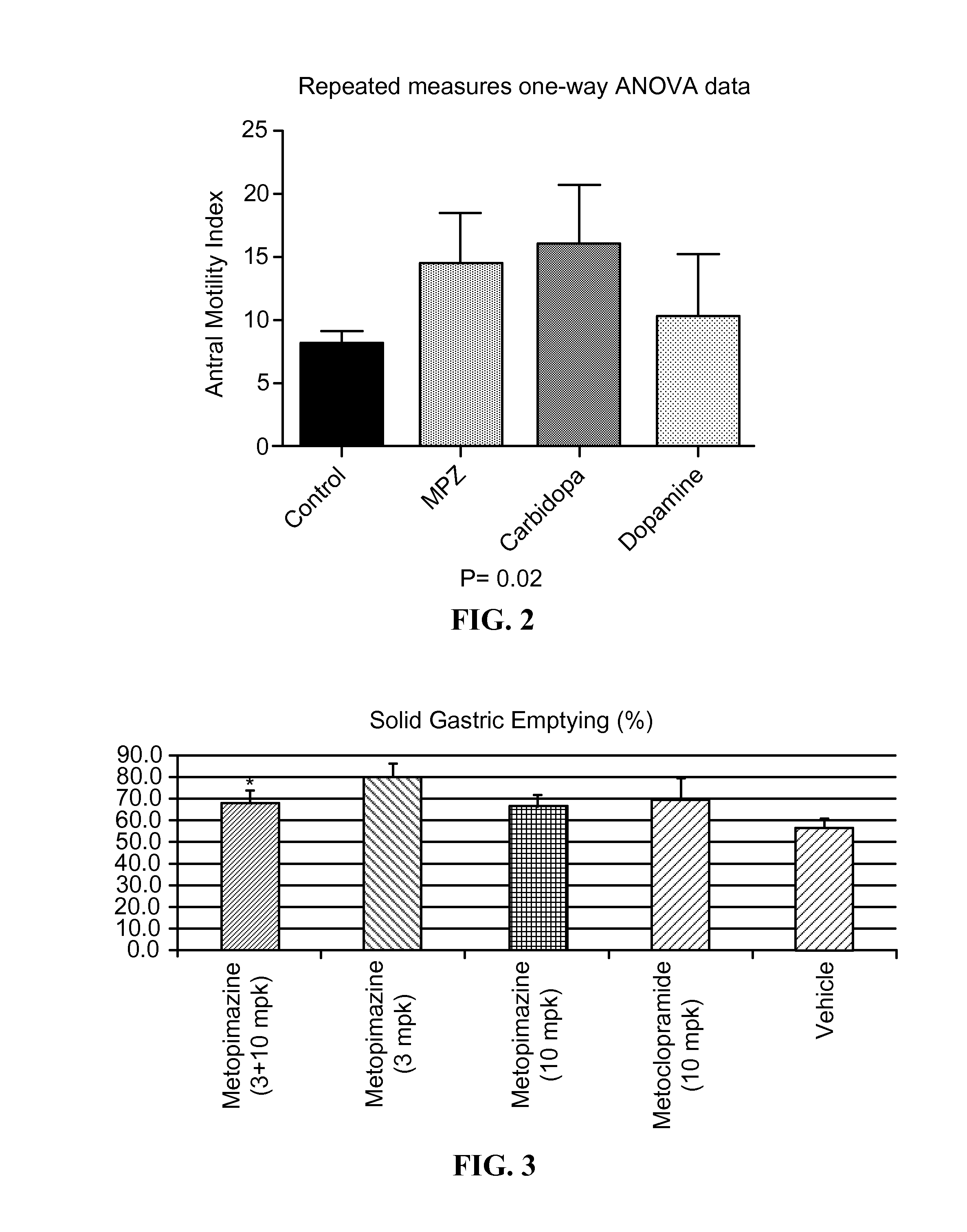
ActiveUS20150087638A1Improving gastric emptyingAntibacterial agentsNervous disorderDiseaseNervous system
Provided herein are methods, compositions, and kits for the treatment of an enteric nervous system disorder. Such methods may comprise administering to a subject an effective amount of a phenothiazine compound, a peripherally restricted dopamine decarboxylase inhibitor, and / or a peripherally restricted dopamine D2 receptor antagonist that does not substantially inhibit hERG channels.
Owner:NEUROGASTRX
Compound with analgesic activity, and pharmaceutical applications thereof
The present invention provides compounds of general formula I, isomers thereof, non-toxic pharmaceutically acceptable salts or solvates thereof. The compounds have remarkably strengthened analgesic activity, reduce the acute toxicity obviously, have no inhibition to heart Herg channel, and reduce side effects on coordination movement of mice remarkably. The present invention realizes the separation of activity and side effects, and has a good application prospect.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Compositions and methods for performing reverse gene therapy
InactiveUS7282489B2Reduce diseaseReduce disorderOrganic active ingredientsBacteriaPlasmid VectorGene product
The invention relates to compositions and methods for reverse gene therapy, wherein a gene therapy vector encoding a gene product (e.g. a protein) which is usually only expressed in cells of an abnormal tissue is delivered to a cell of an animal afflicted with a disease or disorder to alleviate the disease or disorder. In one embodiment, a plasmid vector encoding HERG (A561V) protein is delivered to a cell of an animal afflicted with re-entrant atrial flutter-mediated cardiac arrhythmia.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
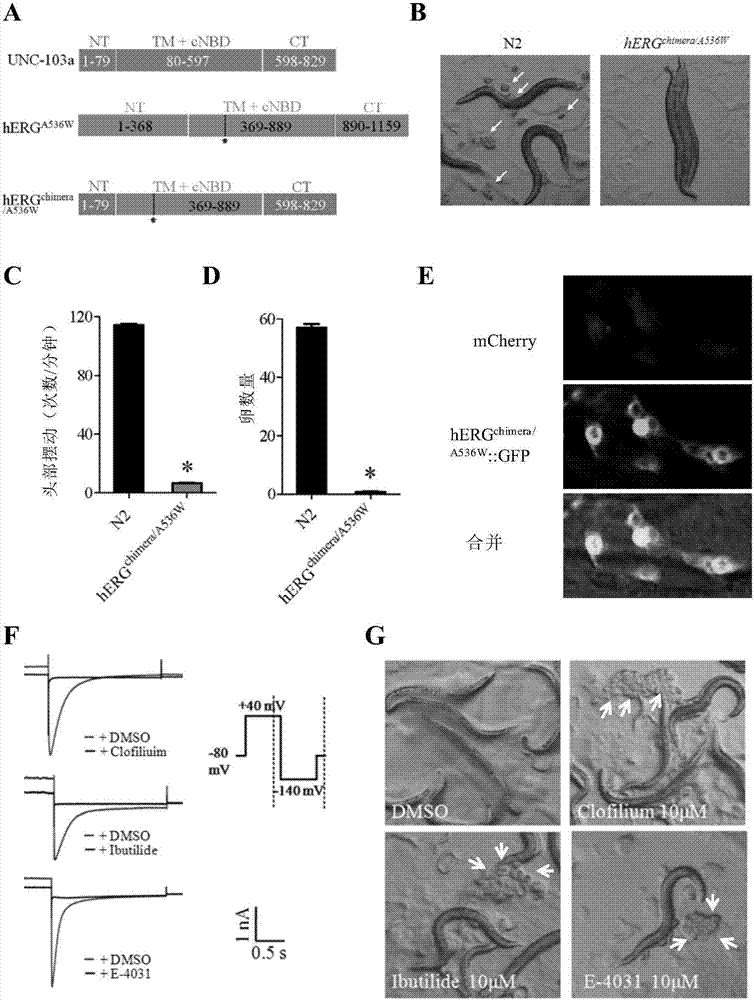
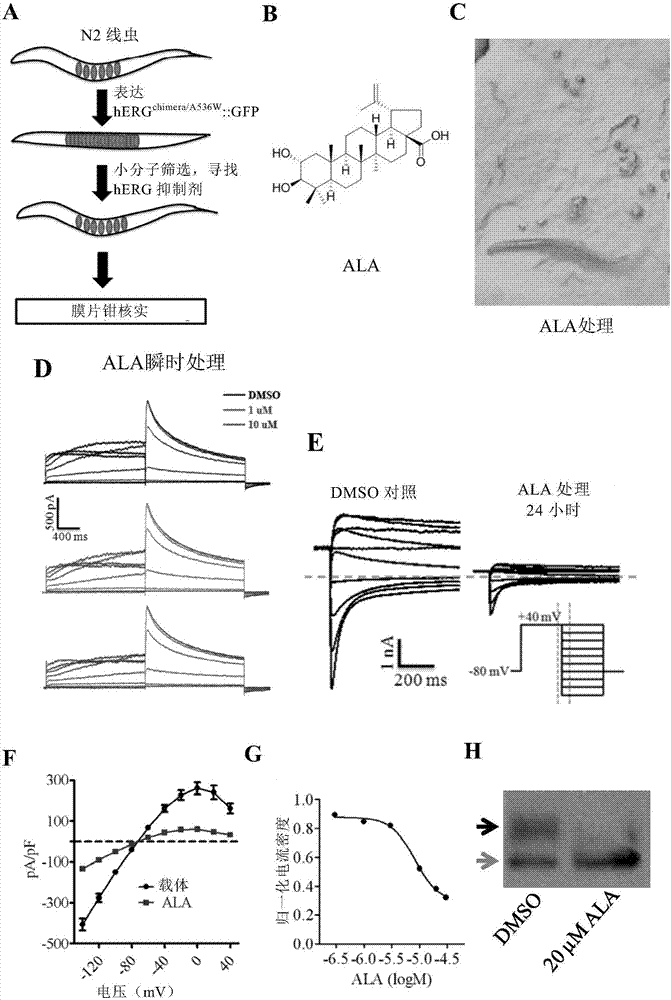
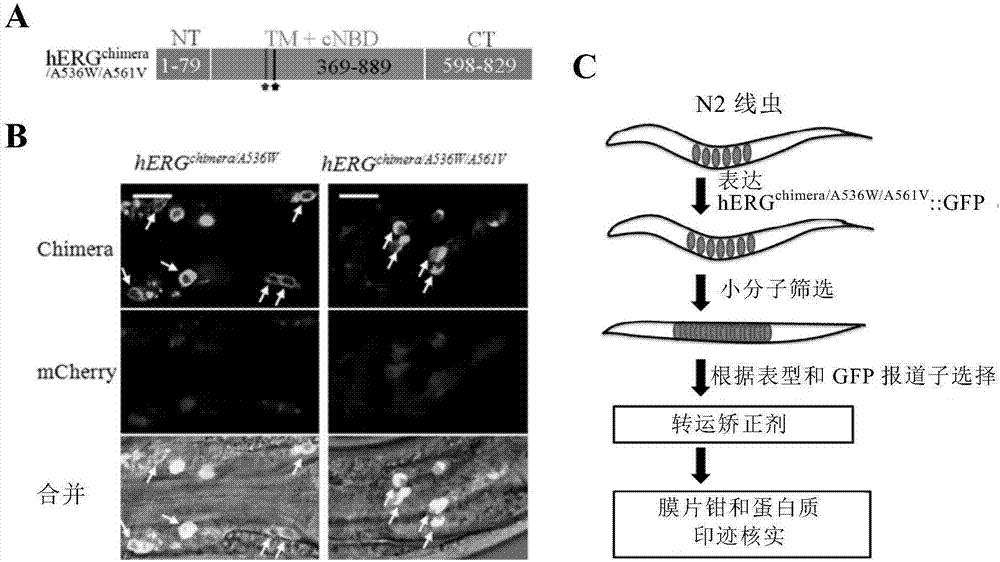
Method for screening hERG potassium ion channel agonist and detecting toxicity
ActiveCN107011444APolypeptide with localisation/targeting motifBiological testingCyclic nucleotide bindingScreening method
The invention relates to a method for screening hERG potassium ion channel agonist and detecting toxicity. Particularly, the invention firstly relates to a fusion protein, which contains a fragment (which is used as the N end of the fusion protein, is from a position between 1st to 85th amino acid residues of the N end of the nemathelminth ERG family potassium ion channels UNC-103 protein and has the length of 75 to 85 amino acid residues), hERG or a fragment at least containing S1-S6 transmembrane domains and cyclic nucleotide binding domains, and a fragment (which is used as the C end of the fusion protein, is from a position between 590th to 829th of amino acid residues of the C end of the UNC-103 protein and has the length of 220 to 240 amino acid residues). The invention also relates to a polynucleotide sequence coding the fusion protein, a relevant transgenic nemathelminth, a relevant screening method and application. The inventor builds an in-vivo hERG-intracellular-transport-influence-recognizable compound molecule screening method for screening hERG inhibitors or LQTS (long QT syndrome) relevant channel mutant functional correcting agents for the first time; the novel path and method are provided for hERG toxicity detection and LQTS treatment medicine screening.
Owner:CENT FOR EXCELLENCE IN BRAIN SCI & INTELLIGENCE TECH CHINESE ACAD OF SCI
Compositions and methods for performing reverse gene therapy
InactiveUS20090068745A1Reduce diseaseReduce disorderBiocideOrganic active ingredientsPlasmid VectorGene product
The invention relates to compositions and methods for reverse gene therapy, wherein a gene therapy vector encoding a gene product (e.g. a protein) which is usually only expressed in cells of an abnormal tissue is delivered to a cell of an animal afflicted with a disease or disorder to alleviate the disease or disorder. In one embodiment, a plasmid vector encoding HERG (A561V) protein is delivered to a cell of an animal afflicted with re-entrant atrial flutter-mediated cardiac arrhythmia.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
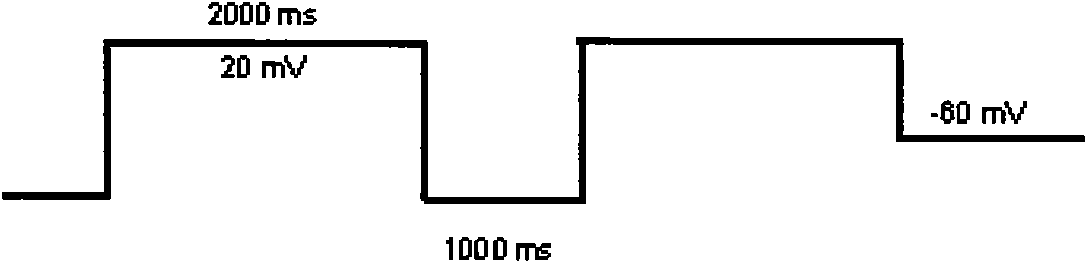
Test method for evaluating arrhythmia caused by action of medicament on human nether-a-gogo related gene (hERG)
InactiveCN102253084AAvoid huge lossesSimple processMaterial analysis by electric/magnetic meansIonHUMAN ETHER-A-GO-GO-RELATED GENE
The invention relates to a test method for evaluating arrhythmia caused by action of medicament on a human nether-a-gogo related gene (hERG). The test method is characterized by comprising the following steps of: depolarizing cells to 40 mv / 5ms conventionally to activate an hERG ion channel; returning thin pliers to -50 mv / s to record the deactivating process of the channel, wherein the maximum current recorded at -50 mv is the hERG ion channel current amplitude required to be recorded; performing a voltage clamp flow test to acquire the speed of the medicament separated from the channel; calculating the time dependence of the compound hERG ion channel at 500 ms and 2 s by inhibition concentration 50150 (IC50150) ms / IC50500ms and IC50150ms / IC502s, wherein if the ratio is high, the time dependence is high; and on the premise of determining that the medicament has remarkable time dependence in the blocking hERG ion channel test, calculating the recovery time index of the medicament hERG blocking agent by a formula of RRec equal to (IC150ms-2-IC502s) / IC150150ms-IC502s), wherein the medicament with the recovery time index of less than 25 is determined to have higher possibility of inducing TdP.
Owner:上海灏远生物医药科技有限公司
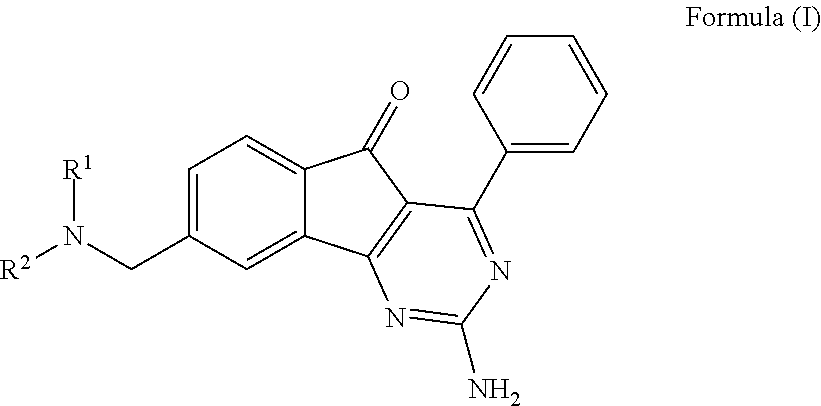
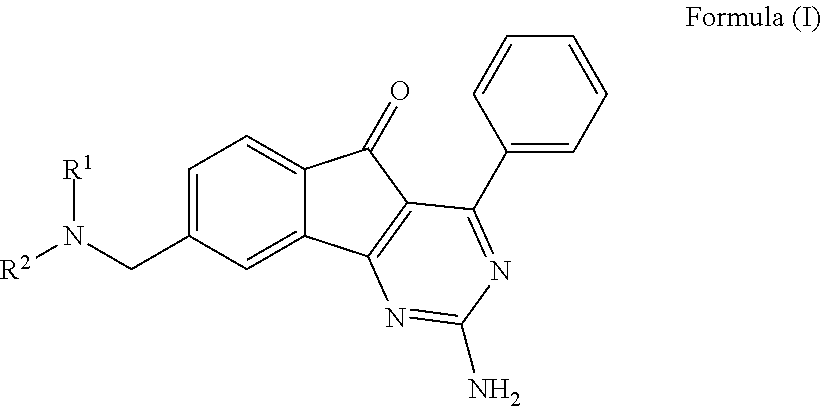
ARYLINDENOPYRIMIDINES WITH REDUCED hERG CHANNEL BINDING
InactiveUS20110312956A1Risk minimizationAmeliorate any conditionBiocideOrganic active ingredientsAdenosineHUMAN ETHER-A-GO-GO-RELATED GENE
This invention provides novel arylindenopyrimidines of the Formula (I),and pharmaceutical compositions comprising same, useful for treating disorders ameliorated by antagonizing adenosine A1 and / or A2a receptors. This invention also provides therapeutic and prophylactic methods using the instant pharmaceutical compositions.
Owner:JANSSEN PHARMA NV
Cardiomyocyte-like cell clusters derived from hBS cells
A cluster comprising cardiomyocyte-like cells, wherein the cluster has i) contracting cells, ii) cells that are electrically connected, and expresses iii) cardiac markers including Nkx.2.5, troponin and myosin, iv) markers for functional adrenergic receptors, v) markers for functional muscarinic receptors, vi) markers for functional ion-channels including hERG, Na+, Ca2+ and K+ channels, vii) one or more endodermal markers selected from the group consisting of AFP, TF, APOA2, AHSG, SERPINA1, APOA1, APOC3, TTR1 APOB, and RBP4. A method for preparing the clusters and the use of the clusters in drug discovery and toxicity screenings are described.
Owner:CELLECTIS SA
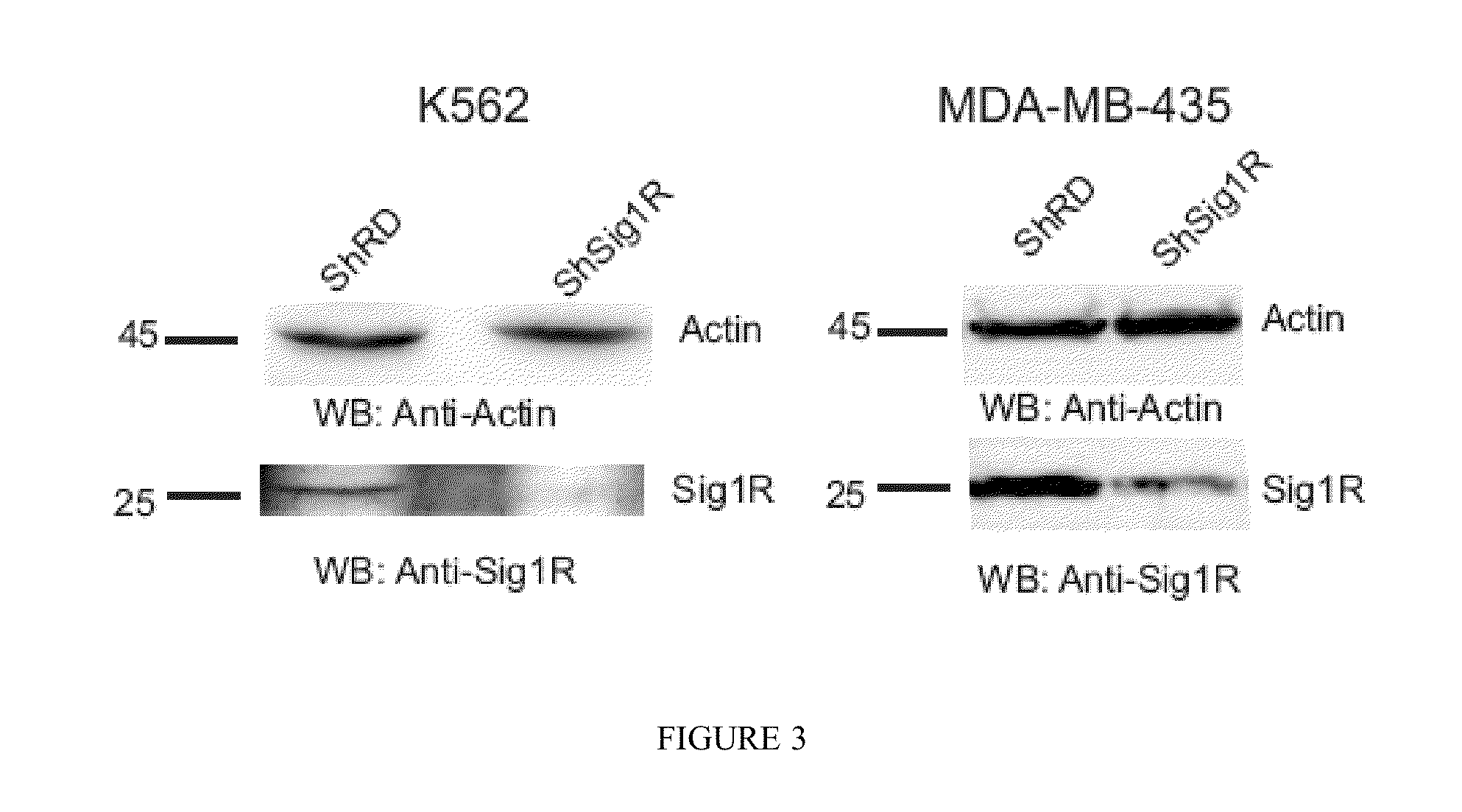
INHIBITORS OF THE INTERACTION OF THE SIGMA-1 RECEPTOR WITH hERG FOR USE IN THE TREATMENT OF CANCER
InactiveUS20130317226A1Modulate the maturation and the membrane stability of hERGNervous disorderOrganic chemistryMedicineSigma-1 receptor
The present invention relates to the use of the Sigma-1 receptor (Sig1R) in the context of the post-transcriptional regulation of the membrane expression of ion channels.The present invention can be used in the field of the treatment of diseases involving ion channels. These are, for example, nervous system diseases, neurodegenerative diseases, heart diseases, and cancer.
Owner:CENT NAT DE LA RECHERCHE SCI +1
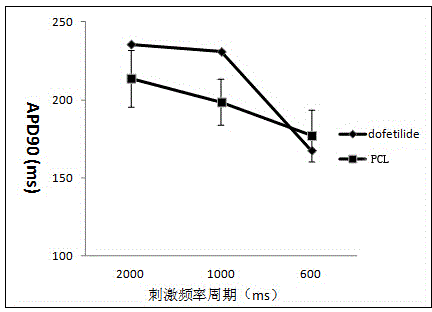
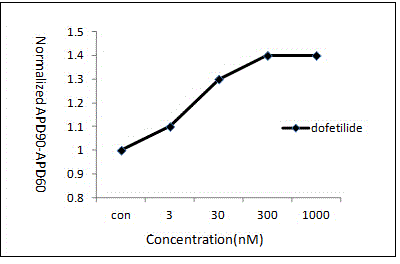
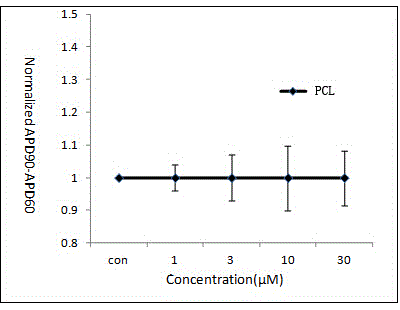
Pharmaceutical pre-clinical cardiac risk assessment method
InactiveCN104951870AImprove screening efficiencyImprove accuracyResourcesTriangulationPharmaceutical care
The invention provides a pharmaceutical pre-clinical cardiac risk assessment method. The method includes the steps of design of action potential frequency, recording of action potential, and recording of a manual patch clamp for the hERG (human ether-Alpha-go-go-related gene) channel. The method has the advantages that the risk of pharmaceutical cardiac arrhythmias is assessed by means of frequency dependence, action potential triangulation and hERG blocking dynamics, screening efficiency and accuracy of pharmaceuticals is greatly improved, and pharmaceutical clinical cardiac arrhythmias is less false positive.
Owner:NANTONG PHARMACORE LABS MEDICAL TECH
Herg channel-expressing cell
InactiveUS20060292546A1High expressionSensitive highCell receptors/surface-antigens/surface-determinantsSugar derivativesEukaryotic plasmidsViral vector
It is an object of the present invention to establish a method of establishing a cell with a remarkably high hERG channel expression level for use in predicting adverse effects based on hERG channel inhibition in research and development of drugs, and to thereby establish a highly sensitive and high throughput evaluation method. By inserting a hERG gene into a retrovirus vector plasmid or lentivirus vector plasmid to thereby prepare a virus vector, concentrating the vector by ultracentrifugation if necessary, transferring the hERG gene into cells and expressing hERG channels therein, it has become possible to secure an expression level effective in measurements using a fully automated high throughput patch clamp system or dyes.
Owner:EISIA R&D MANAGEMENT CO LTD
Application of AMO-143 in preparation of medicines for treating long QT syndrome
InactiveCN105169414AOrganic active ingredientsGenetic material ingredientsLong QT syndromeHUMAN ETHER-A-GO-GO-RELATED GENE
The invention discloses an application of AMO-143 in preparation of a pharmaceutical composition for preventing and treating a long QT syndrome and promoting hERG expression, and the pharmaceutical composition for treating the long QT syndrome. The pharmaceutical composition is characterized by containing the AMO-143.
Owner:NINGBO MEDICAL CENT LIHUILI HOSPITACL
Methods for determining precise HERG interactions by mutagenesis
InactiveUS20060084102A1Avoid and eliminate cardiac liabilityReduce and avoid undesirable interactionCell receptors/surface-antigens/surface-determinantsSugar derivativesHeterologousIn vivo
Owner:NEURION PHARMA INC
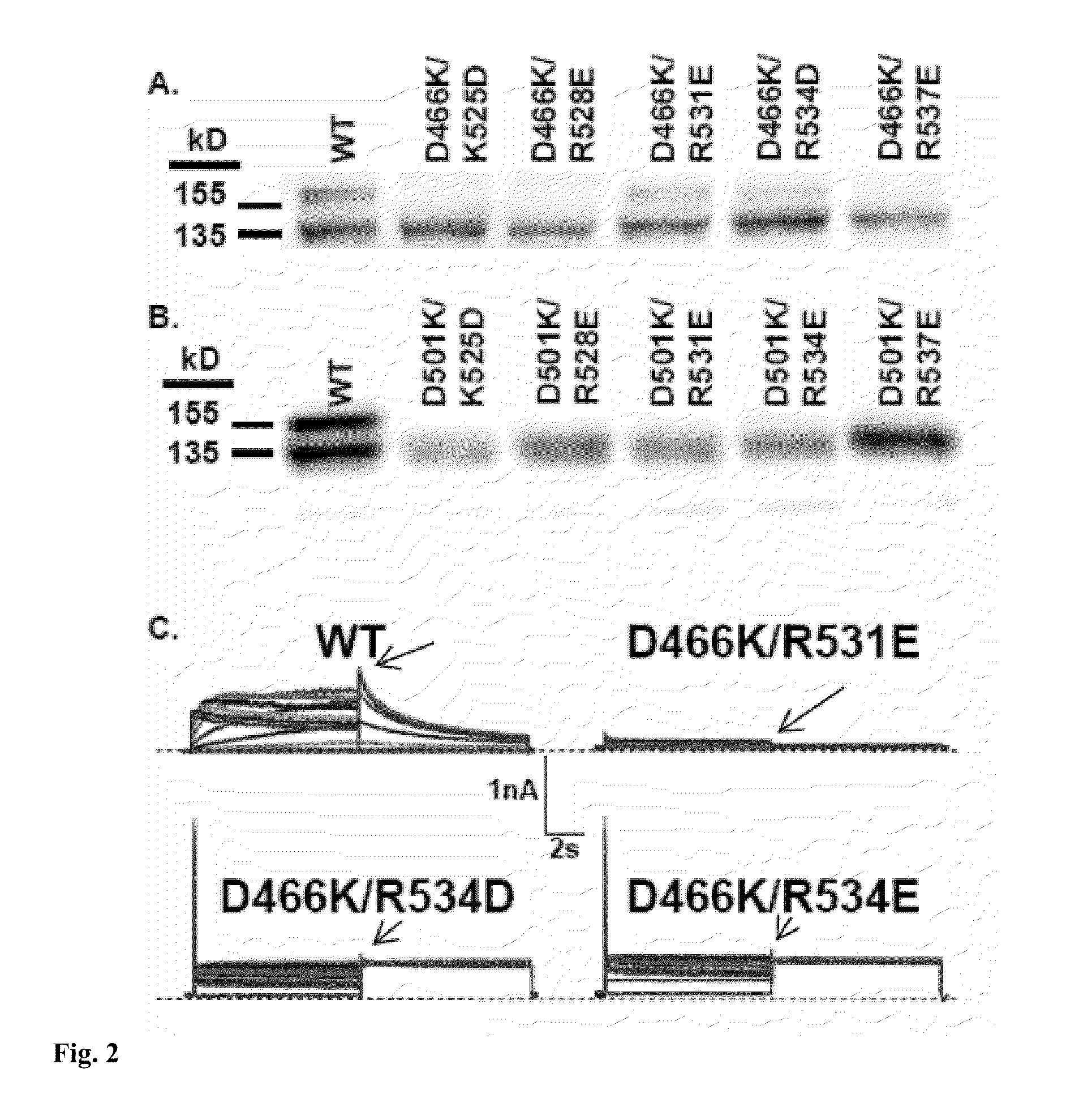
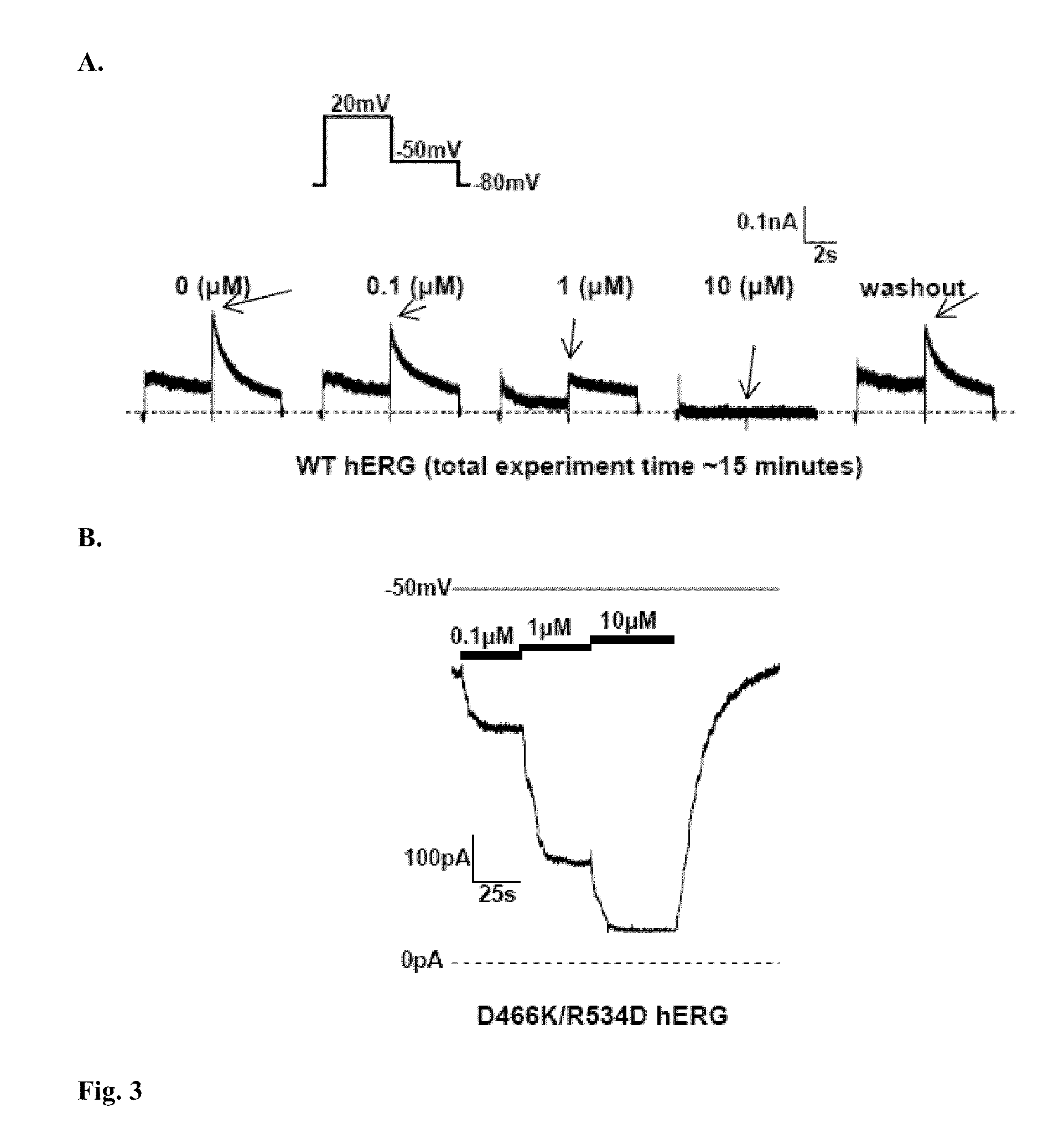
Constitutively open hERG (Kv11.1) mutant channels
InactiveUS7820437B2Compound screeningCell receptors/surface-antigens/surface-determinantsDouble mutationAmino acid substitution
We disclose a cell having double mutations of the hERG gene that lead to charge reversal amino acid substitutions at residues 466 and 534 of the wild type Kv11.1 channel protein. These double charge reversal mutations result in cells having constitutively open Kv11.1 channels. Such cells could be used in a method of testing development-stage drugs and other compounds for Kv11.1 channel block activity.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com