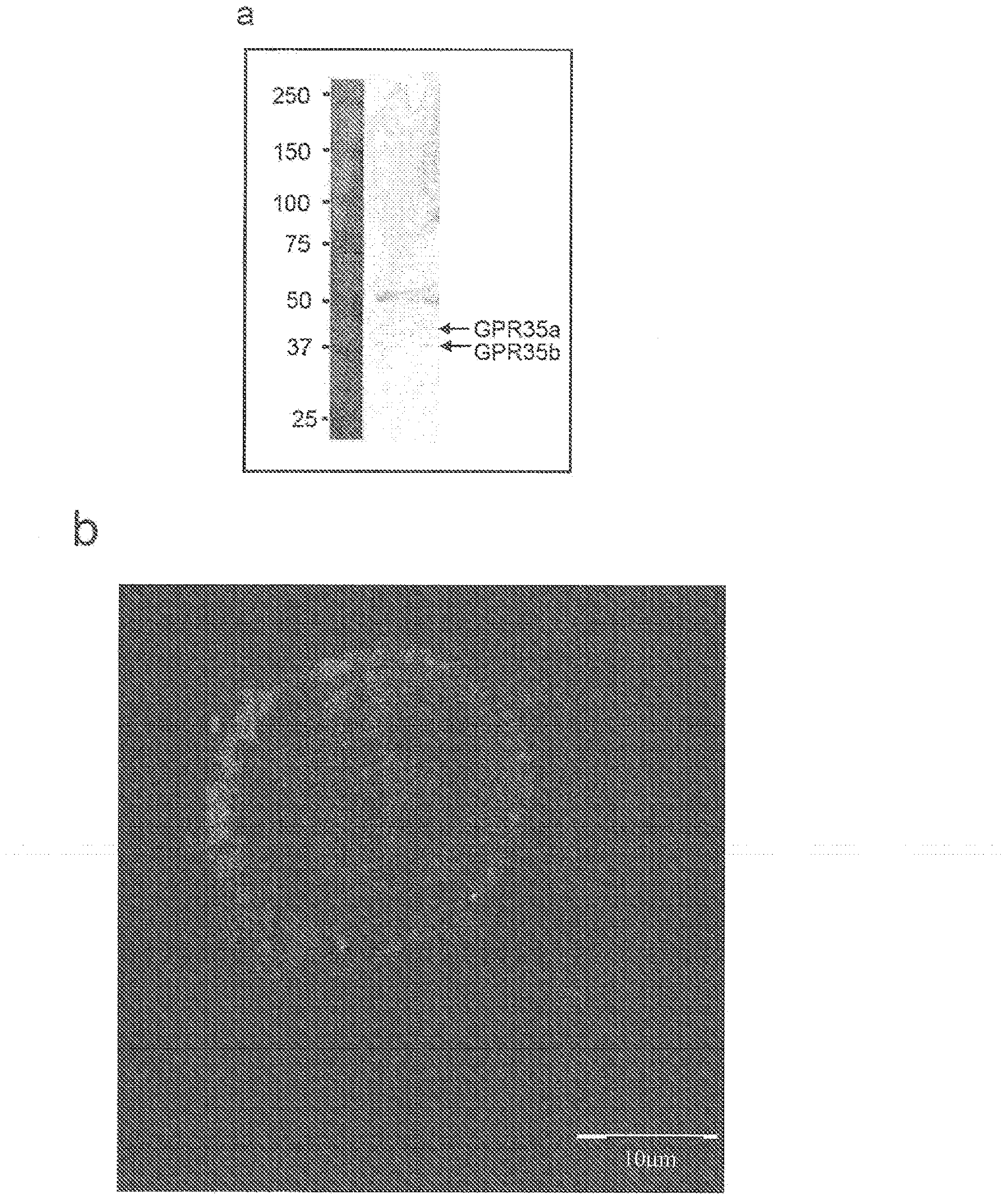
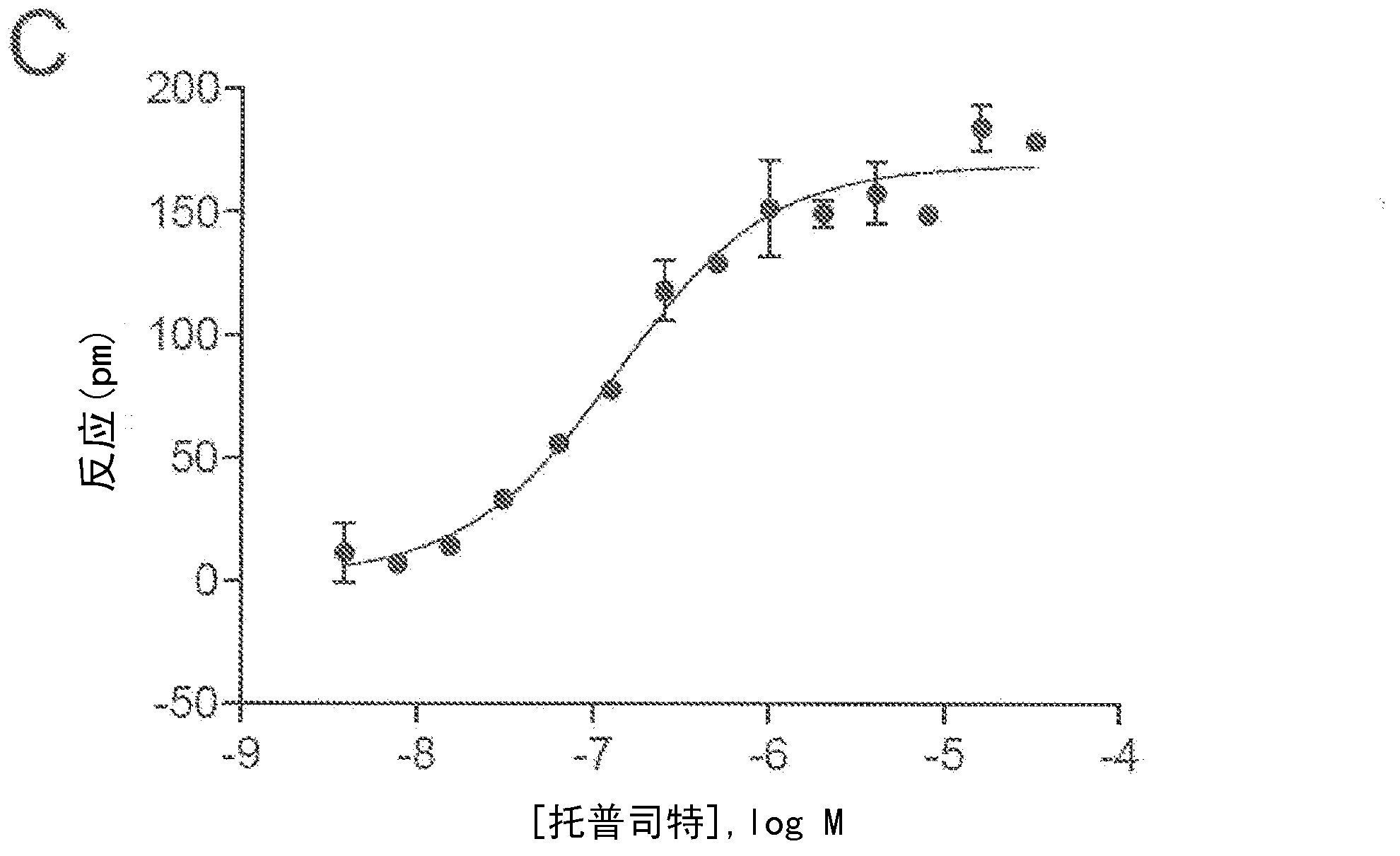
Compositions and methods for the treatment of pathological condition(s) related to GPR35 and/or GPR35-HERG complex
The technology of a compound, 2NR101R102, is applied in the field of compositions and methods for treating pathological conditions related to GPR35 and/or GPR35-HERG complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0686] 1. Preparation of compounds of formula (I), (II), (III), (IV), (V) and (VI)
[0687] The following are examples of the preparation of compounds of formula (I), (II), (III), (IV), (V) and (VI). This embodiment is exemplary only, and is not intended to limit the present disclosure.
[0688]
[0689] i.2-Dicyanomethylene-3-cyano-4,5-dimethyl-5-[4’-n-butylphenyl]-2,5-dihydrofuran
[0690] 3-Hydroxy-3-[4'-n-butylphenyl]-2-butanone 10.0g (0.0455 mol), malononitrile 6.0g (0.091 mol), lithium ethoxide 2.3ml (2.3mmol) and THF50ml Mix and boil at reflux overnight. The pure product (2-dicyanomethylene-3-cyano-4,5-dimethyl-5-[4'-n-butylphenyl]-2,5-dihydrofuran) was obtained from ethanol Recrystallization of to generate 9.95g: 69.1% yield.mp: 129.3-130.5°C. 1H NMR: δ7.259(d,2H),7.112(d,2H),2.638(t,2H,CH 2 ),2.225(s,3H,Me),1.997(s,3H,Me),1.60-1.28(m,4H,CH 2 ),0.931(t,3H,CH 3 ). 13 C NMR: 182.476, 175.753, 145.841, 131.069, 129.692(2C), 125.075(2C), 110.914, 110.406, 109.08...
Embodiment 2
[0786] 1. Materials and Methods
[0787] i.Material
[0788] Toprinast was obtained from BioMol International Inc (Plymouth Meeting, PA). cell culture compatibility 384 Biosensor Microplates were obtained from Corning Incorporated (Corning, NY).
[0789] Both HEK293 and HT-29 cells were obtained from American Type Cell Culture (Manassas, VA). Cell culture media were as follows: (1) Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS), 4.5 g / L glucose, 2 mM glutamine, and antibiotics for HEK293; and (2) McCoy' s5a modified medium supplemented with 10% FBS, 4.5 g / L glucose, 2 mM glutamine, and antibiotics for human colorectal adenocarcinoma HT29.
[0790] The HEK hERG stable cell line (HEK-hERG) and the CHO hERG stable cell line (CHO-hERG) were maintained according to Sun et al. (J. Biol. Chem. 2006, 281:5877). Cells were subcloned 1-2 times per week, and cells with less than 15 passages were used for all experiments.
[0791] ii. Calcium flux test
[0792...
Embodiment 3
[0835] 1. Materials and Methods
[0836] i.Material
[0837] Toprinast was obtained from BioMol International Inc (Plymouth Meeting, PA). cell culture compatibility 384 Biosensor Microplates were obtained from Corning Incorporated (Corning, NY). All tyrphostin compounds were obtained from Sigma Chemical Co. (St. Louis, MO).
[0838] Both HEK293 and HT-29 were obtained from the American Type Cell Culture (Manassas, VA). Cell culture media were as follows: (1) Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS), 4.5 g / L glucose, 2 mM glutamine, and antibiotics for HEK293; and (2) McCoy' s5a modified medium supplemented with 10% FBS, 4.5 g / L glucose, 2 mM glutamine, and antibiotics for human colorectal adenocarcinoma HT29.
[0839] ii. Calcium flux test
[0840] Fluo-4Direct TM Calcium assay kit was purchased from Invitrogen (Starter pack, catalog number F10471). HEK293 cells (15,000 cells / well) and HT29 cells (30,000 cells / well) were seeded into poly-D-l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com