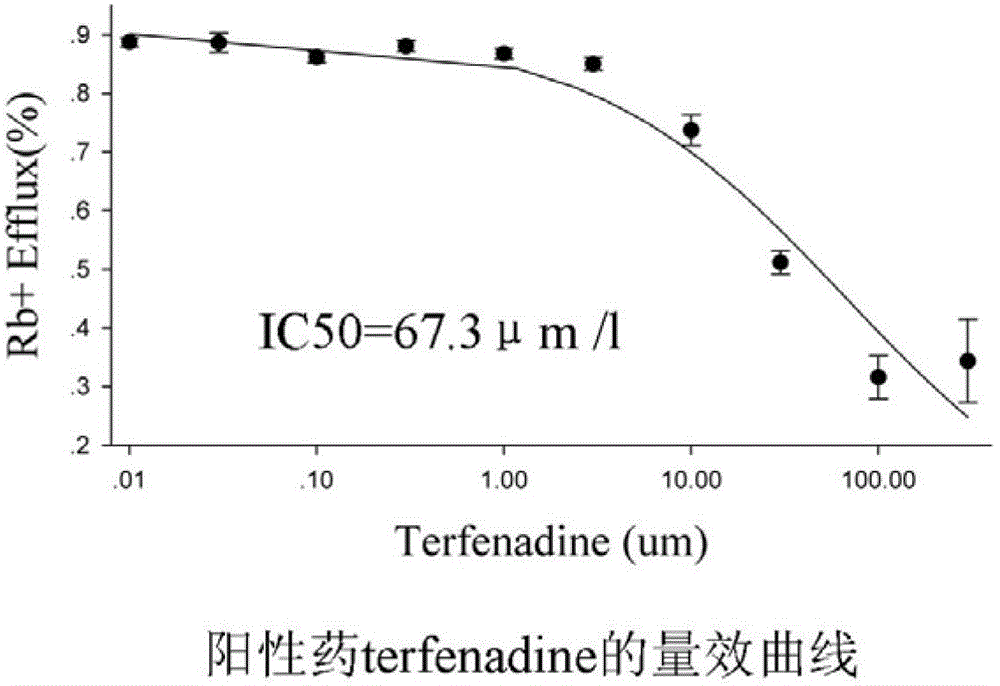
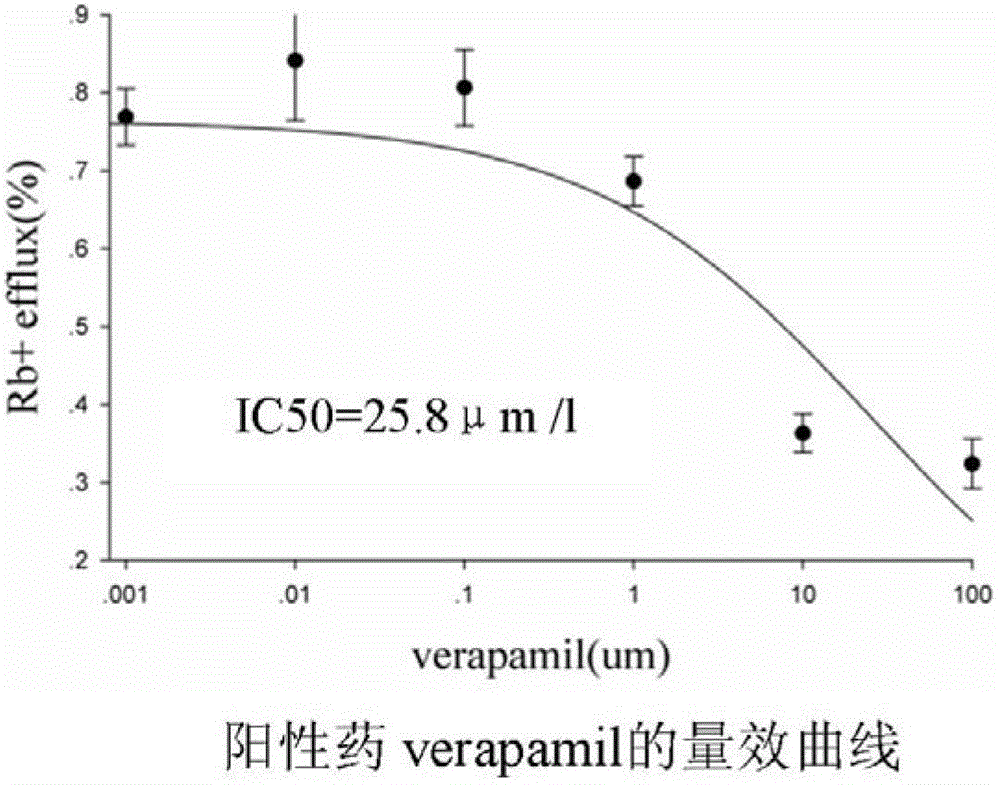
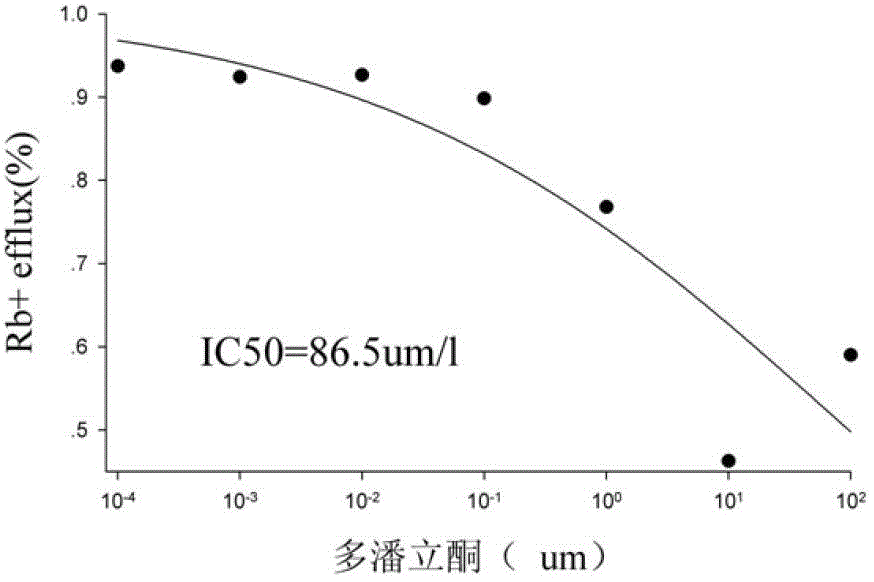
Method for efficiently evaluating safety of medicine in hERG channel
An evaluation method and drug technology, applied in the medical field, can solve problems such as high requirements for technicians, expensive experimental equipment, and low efficiency of hERG channel safety evaluation, and achieve the effects of improved efficiency, faster screening, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Cell culture: CHO cell lines stably expressing hERG channels (existing in the prior art) were inoculated in Hams F-12 medium, at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Routine cultivation in an incubator.
[0033] (2) Seed plate: Take cell lines in good condition and the degree of polymerization reaches 90% and plant them in a 96-well plate at a density of 50,000 per well. 2 under the condition of CO 2 Routine cultivation in an incubator.
[0034] (3) Fixed Rb + : When the cells grow to cover about 90% of the bottom of the well, discard the culture medium in the well, add 200 μL of fixation buffer, and store at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Incubate in the incubator for 2.5h.
[0035] (4) Drug treatment: Discard the immobilization buffer in each well, add the drug to be tested prepared with the immobilization buffer, and incubate for 30 min.
[0036] (5) Washing: discard the im...
Embodiment 2
[0049] (1) Cell culture: CHO cell lines stably expressing hERG channels (existing in the prior art) were inoculated in Hams F-12 medium, at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Routine cultivation in an incubator.
[0050] (2) Seed plate: Take cell lines in good condition and the degree of polymerization reaches 90% and plant them in a 96-well plate at a density of 50,000 per well. 2 under the condition of CO 2 Routine cultivation in an incubator.
[0051] (3) Fixed Rb + : When the cells grow to cover about 90% of the bottom of the well, discard the culture medium in the well, add 200 μL of fixation buffer, and store at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Incubate for 1 h in the incubator.
[0052] (4) Drug treatment: discard the immobilization buffer in each well, add the drug to be tested prepared with the immobilization buffer, and incubate for 40 min.
[0053] (5) Washing: discard the imm...
Embodiment 3
[0066] (1) Cell culture: CHO cell lines stably expressing hERG channels (existing in the prior art) were inoculated in Hams F-12 medium, and incubated at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Routine cultivation in an incubator.
[0067] (2) Seed plate: Take cell lines in good condition and the degree of polymerization reaches 90% and plant them in a 96-well plate at a density of 50,000 per well. 2 under the condition of CO 2 Routine cultivation in an incubator.
[0068] (3) Fixed Rb + : When the cells grow to cover about 90% of the bottom of the well, discard the medium in the well, add 200 μL of fixation buffer, and store at 37°C, saturated air humidity, 95% air + 5% CO 2 under the condition of CO 2 Incubate for 4 hours in the incubator.
[0069] (4) Drug treatment: Discard the immobilization buffer in each well, add the drug to be tested prepared with the immobilization buffer, and incubate for 5 min.
[0070] (5) Washing: Discar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com