Test method for evaluating arrhythmia caused by action of medicament on human nether-a-gogo related gene (hERG)
A test method and arrhythmia technology, applied in measuring devices, material analysis through electromagnetic means, instruments, etc., can solve problems such as poor TdP correlation, high TdP false positive rate, loss of pharmaceutical companies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The present invention is further described below in conjunction with the examples.
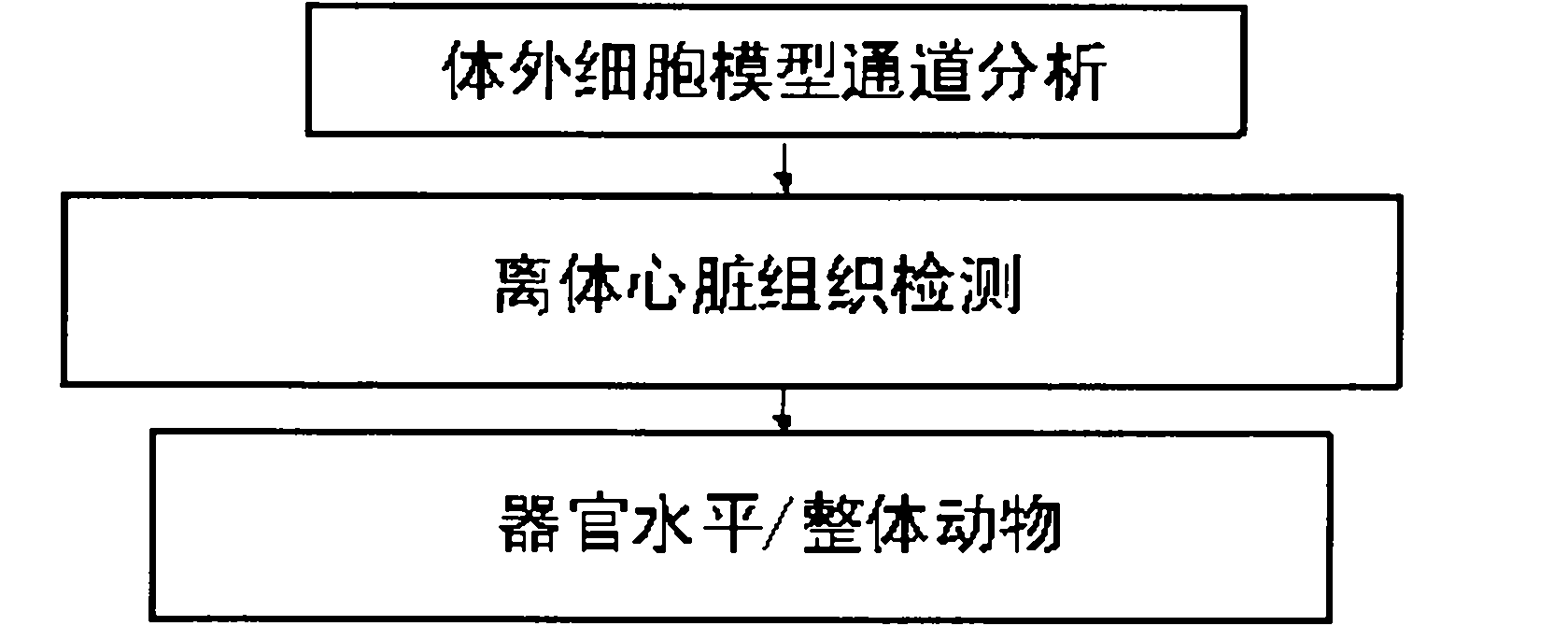
[0021] The test method proposed by the present invention for assessing the arrhythmia caused by the action of drugs on hERG is a more comprehensive and more accurate innovative solution to the methods commonly used in the world at present. The test method includes an in vitro cell model channel Analysis, isolated heart tissue detection and organ level / whole animal three processes, characterized in that: the channel analysis of the extracellular cell model takes the following steps:
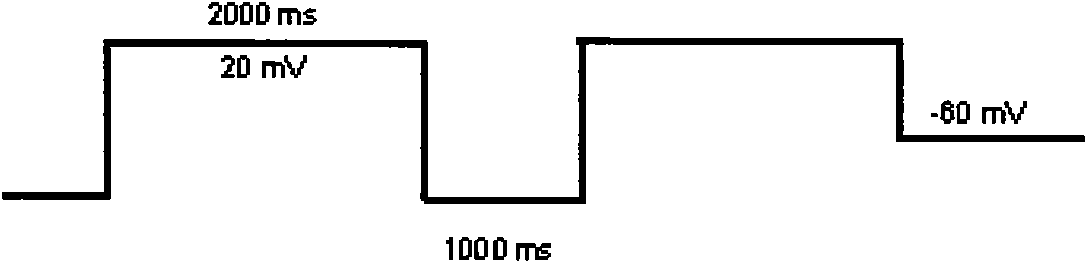
[0022] 1) Depolarize the cell to 40mv / 5ms to activate the hERG ion channel according to the routine, and then clamp the cell back to -50mv / s to record the inactivation process of the channel. The maximum current recorded at -50mv is the one to be recorded hERG channel current amplitude;
[0023] 2) Perform a voltage clamp test to obtain the speed at which the drug leaves the channel; this test includes two...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com