Micro-molecule fluorescent probe of phenyl furan hERG potassium channel and application thereof
A technology of potassium ion channels and fluorescent probes, which is applied in the direction of fluorescence/phosphorescence, luminescent materials, analytical materials, etc., can solve the problems of lagging development of biological and physiological properties, and achieve the effect of reducing false positive results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: (E)-2-(5-allyl-2,7-dichloro-4-((4-(4-(3-(((5-(4-chlorophenyl)furan- 2-yl)methylene)amino)-2,5-dioximidazol-1-yl)butyl)piperazin-1-yl)methyl)-6-hydroxy-3-oxo-3H-oxanthene -9-yl) preparation of benzoic acid
[0035] Concrete synthetic route is as follows:
[0036]
[0037] (1) Preparation of Intermediate 1:
[0038] Add 20mL of distilled water and 14mL of concentrated hydrochloric acid to p-chloroaniline (2.55g, 20mmol), white turbidity appeared, heated to 70°C, completely dissolved; after cooling to 0°C, slowly added dropwise sodium nitrite solution (1.38g dissolved in 10mL Distilled water, 20mmol), after dripping, the reaction solution was clarified, continued to react for 20min, added furfural (2.88g, 30mmol) and copper chloride (0.54g, 4mmol), the reaction solution was green, stirred overnight, and a brown solid was precipitated, which was obtained by suction filtration. Brown-green solid, the filter cake was washed successively with a small amount of...
Embodiment 2
[0055] Example 2: Preparation of naphthalene diimide fluorophore probes
[0056] Concrete synthetic route is as follows:
[0057]
[0058] In the synthetic route of this example, the preparation of intermediates 1-5 is the same as that of intermediates 1-5 in Example 1.
[0059] (1) Preparation of intermediate 7a:
[0060] To 1,8-naphthalene diimide (2.00 g, 10.14 mmol) was added anhydrous K 2 CO 3 (5.59,40.56mmol), 1,3-dibromopropane (6.14g, 30.43mmol) and 20mL of acetonitrile, reflux for 12h. After suction filtration, the filtrate was concentrated and passed through a column to obtain a white solid with a yield of 44.4%. M.p.:140.0-142.0℃. 1 H-NMR (300MHz, CDCl 3 ):δ8.63(dd, J=7.5,1.2Hz,2H),8.24(dd,J=8.1,1.2Hz,2H),7.79(dd,J=8.1,7.5Hz,2H),4.36(t, J=7.2Hz, 2H), 3.53(t, J=6.9Hz, 2H), 2.39-2.29(m, 2H).ESI-MS: ([M+H] + ): 318.2.
[0061] (2) Preparation of intermediate 7b:
[0062] According to the preparation method of intermediate 7a, the difference is: compound 6 ...
Embodiment 3
[0106] Embodiment 3: the mensuration of biological activity
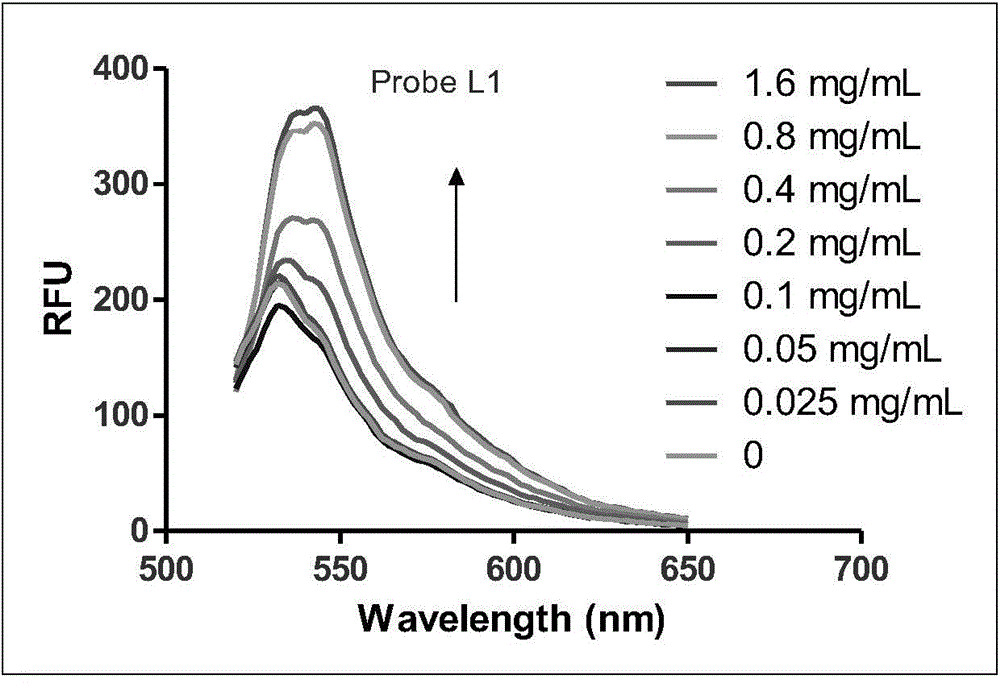
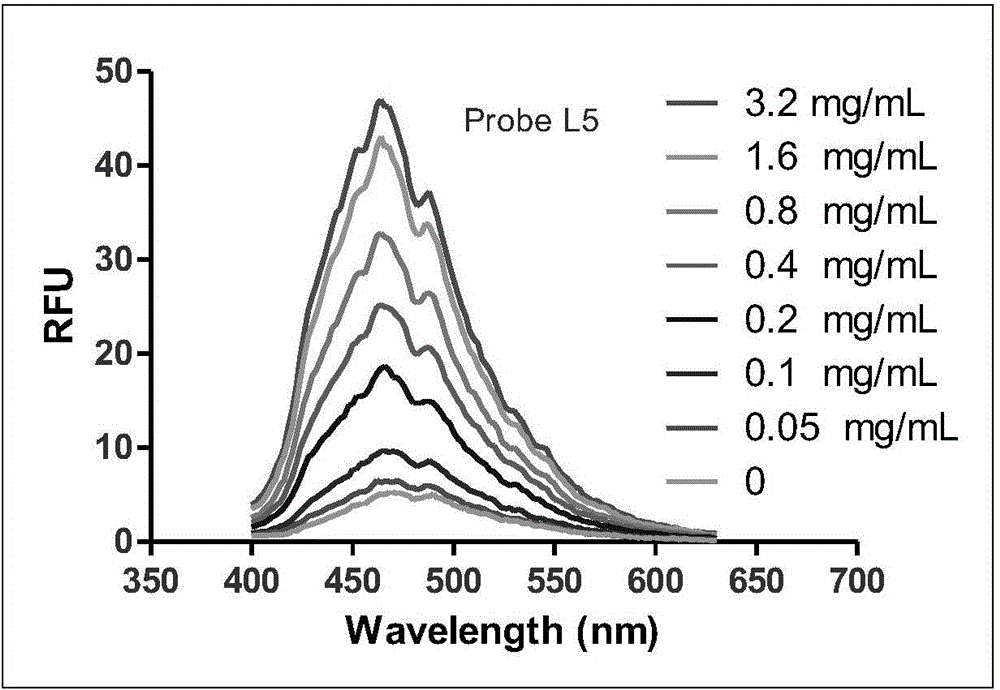
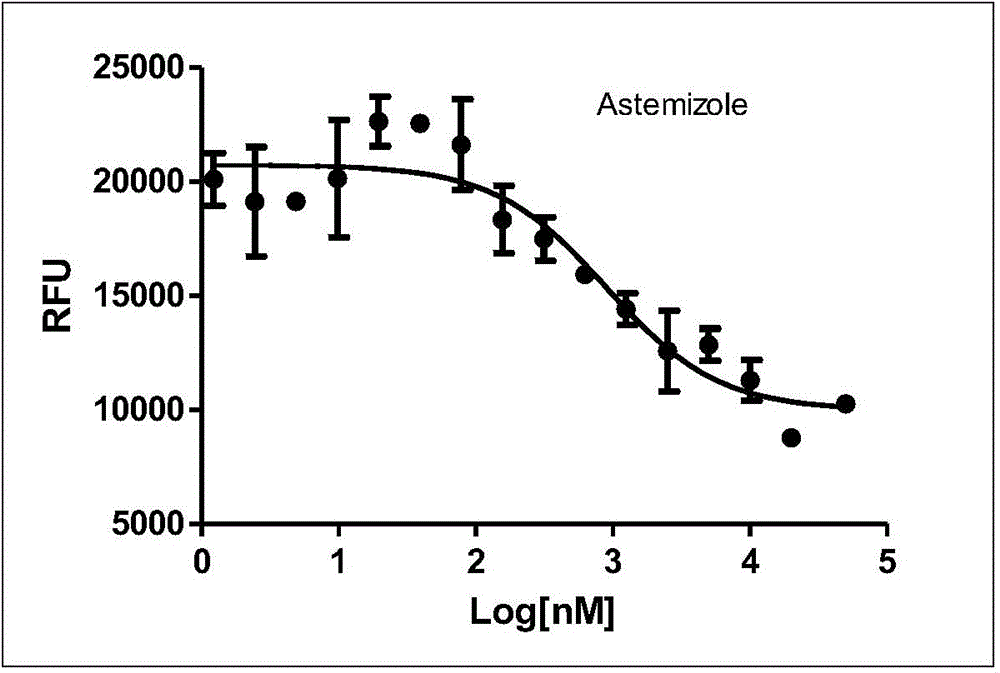
[0107] Azimilide (Azimilide) and astemizole (Astemizole) were used as positive drugs, and a radioligand competitive binding experiment was used (reference: Identification of Human Ether-`a-go-go Related GeneModulators by Three Screening Platforms in an Academic Drug-Discovery Setting), measure the binding activity of fluorescent probe molecule and hERG potassium ion channel, the results are shown in Table 1, the activity of synthetic probe molecule is all improved than recognition group Azimilde, probe molecule and hERG potassium ion channel Have higher activity. However, there is a big difference in the fluorescence properties. The probe molecule L2-4 has no substituents on the naphthalene diimide ring, the fluorescence is weak and the excitation wavelength and emission wavelength are relatively short. Therefore, in further research, we L1 and L5 were selected for further screening and imaging studies.
[0108] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com