Pharmaceutical pre-clinical cardiac risk assessment method
A risk assessment and preclinical technology, applied in instruments, data processing applications, resources, etc., can solve the problems of imprecise experimental design, improve screening efficiency and accuracy, and reduce false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] A preclinical cardiac risk assessment method for a drug, comprising the following steps:
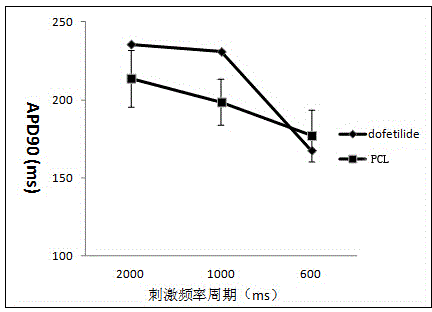
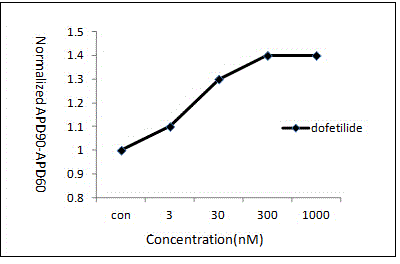
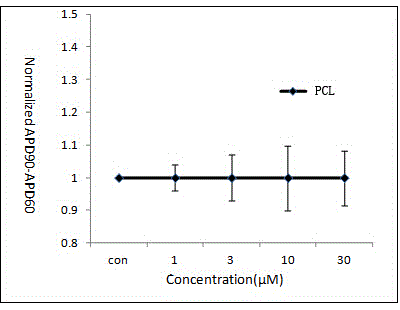
[0018] (1) Action potential frequency design: use three groups of different stimulation frequencies including 100 pulses to stimulate the action potential launched by Purkinje fibers at different stimulation frequencies. The three groups of stimulation frequency periods are 2000ms, 1000ms and 600ms respectively;
[0019] (2) Action potential recording: Place rabbit Purkinje fibers in a perfusion bath, perfuse Tyrode's solution at a rate of 4 mL / min, and record intracellular membrane voltage through intracellular microelectrodes, which are made of borosilicate The salt glass capillary is drawn by an electrode drawing device, and the microelectrode is filled with 3 M KCl inner solution; the intracellular voltage is recorded by the electrometer amplifier connected to the cell through a silver wire; after the voltage is stabilized, the drug is perfused according to the concentration gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com