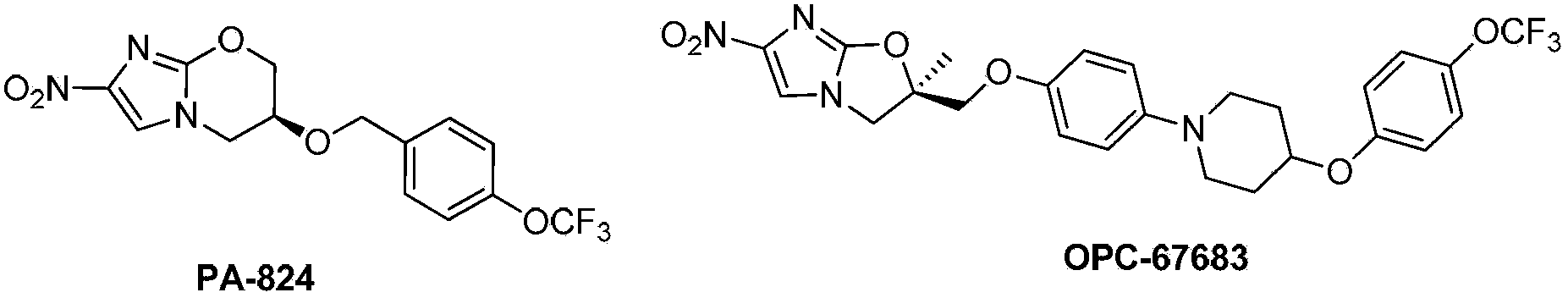
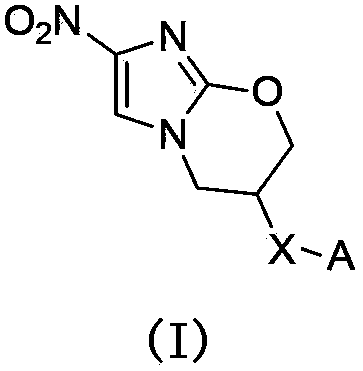
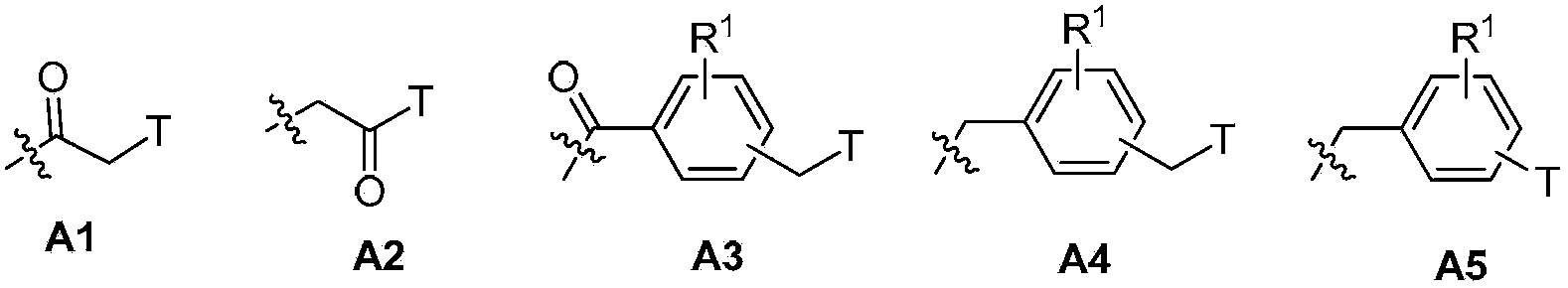
Novel nitroimidazole compound and application thereof in pharmacy
A compound, nitro technology, applied in the fields of pharmacology, medicinal chemistry and pharmacology, can solve the problems of low oral bioavailability, serious adverse reactions, poor effect, etc., to improve the activity of anti-mycobacterium tuberculosis, strong anti- Mycobacterium tuberculosis effect, effect of excellent pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0093] Preparation Example 1 5-(4-(Trifluoromethoxy)phenoxy)isoindole (TH-1)
[0094]
[0095] tert-butyl 5-hydroxyisoindole-2-carboxylate (470 mg, 2.0 mmol, synthesized by reference Bioorg.Med.Chem.Lett., 2001, 11(5), 685-688) and 1-bromo 4-tri Fluoromethoxybenzene (481mg, 2.0mmol) was dissolved in 1,4-dioxane (10mL), copper iodide (38mg, 0.2mmol), N,N-dimethylglycine (62mg, 0.6 mmol), cesium carbonate (977mg, 3.0mmol), heated to reflux for 24h under the protection of argon, the reaction was completed, filtered, concentrated, column chromatography (CH 2 Cl 2 / CH 3 OH=100 / 1), the resulting product is directly put into the next step reaction.
[0096] The intermediate obtained in the previous step was dissolved in dichloromethane (10 mL), and the mixture was cooled with ice salt, and trifluoroacetic acid (10 mL) was added. After the addition was complete, the mixture was reacted at room temperature for 3 hours, stopped the reaction, and concentrated. The residue was dissol...
preparation example 2
[0098] Preparation 2 5-(4-(trifluoromethyl)phenoxy)isoindole (TH-2)
[0099]
[0100] Using tert-butyl 5-hydroxyisoindole-2-carboxylate (470 mg, 2.0 mmol) and 1-bromo-4-trifluoromethylbenzene (450 mg, 2.0 mmol) as starting materials, the intermediate product was obtained similarly to the synthesis of TH-1 TH-2 (340 mg, 61%).
[0101] MS(ESI / LR):280.1[M+1] + .
preparation example 3
[0102] Preparation 3 7-(4-(trifluoromethoxy)phenoxy)-1,2,3,4-tetrahydroisoquinoline (TH-3)
[0103]
[0104] With tert-butyl 7-hydroxy-3,4-dihydroisoquinoline-2(1H)-carboxylate (498mg, 2.0mmol, reference Bioorg.Med.Chem.,2009,17(23),7850-7860 Synthesis) and 1-bromo-4-trifluoromethoxybenzene (481mg, 2.0mmol) as raw materials, similar to the synthesis of TH-1, the intermediate product TH-3 (402mg, 65%) was obtained.
[0105] MS(ESI / LR):310.1[M+1] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com