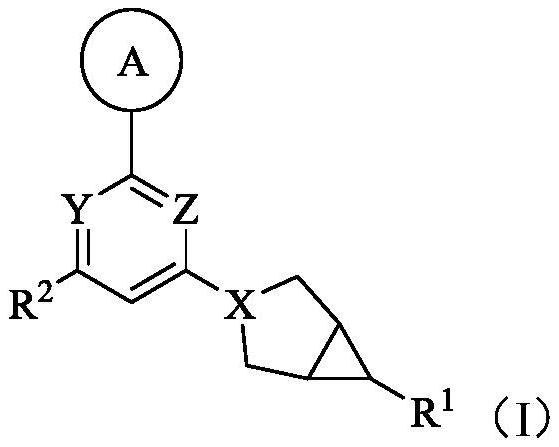
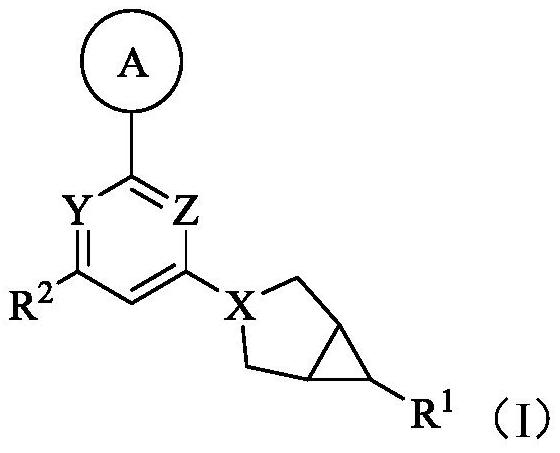
Ketohexokinase (KHK) inhibitor and application thereof
A compound, selected technology, applied in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as unmet clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
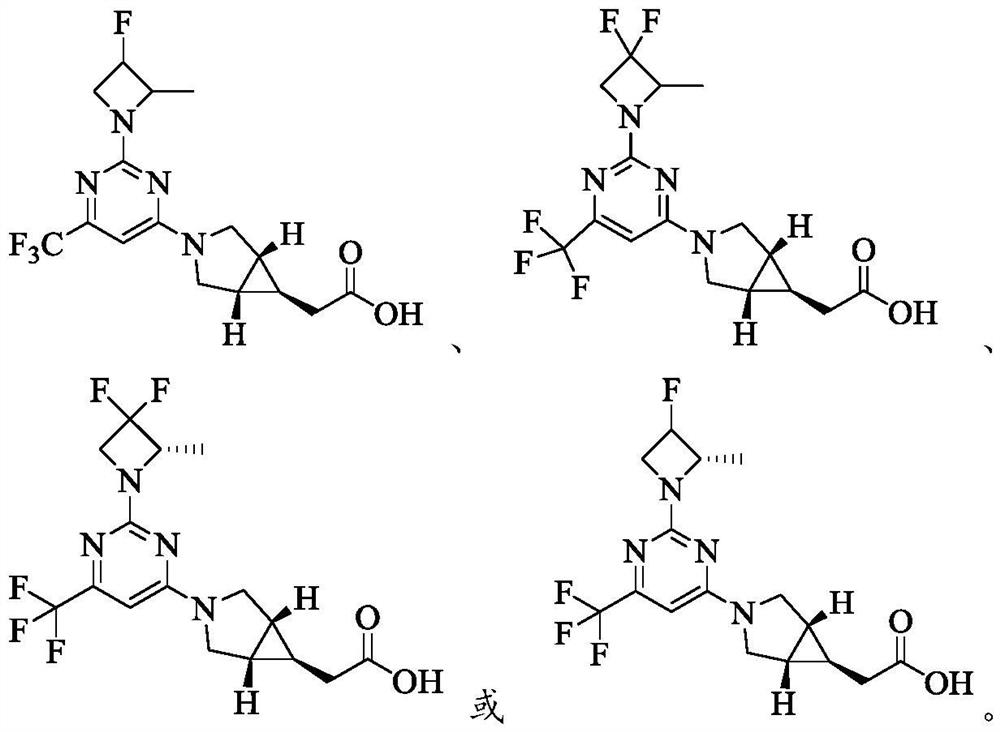
[0133] Example 1 2-((1R,5S,6s)-3-(2-(3-fluoro-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidine-4 Preparation of -yl)-3-azabicyclo[3.1.0]]hex-6-yl)acetic acid (compound 1)
[0134]
[0135] 1) 2-((1R,5S,6s)-3-(2-(3-fluoro-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidine-4- base)-3-azabicyclo[3.1.0]]hex-6-yl)methyl acetate
[0136]
[0137] 2-((1R,5S,6s)-3-(2-chloro-6-(trifluoromethyl)pyrimidin-4-yl)-3-azabicyclo[3.1.0]hex-6-yl) Methyl acetate (50mg, 0.15mmol), 3-fluoro-2-methylazetidine hydrochloride (23mg, 0.18mmol), dissolved in acetonitrile (10mL), added N,N-diisopropyl Ethylamine (0.5 mL), heated to 65°C for 16 hours, spin-dried, and the residue was directly used in the next step.
[0138] 2) 2-((1R,5S,6s)-3-(2-(3-fluoro-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidine-4- base)-3-azabicyclo[3.1.0]]hex-6-yl)acetic acid
[0139]
[0140] 2-((1R,5S,6s)-3-(2-(3-fluoro-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidin-4-yl) -3-Azabicyclo[3.1.0]]hex-6...
Embodiment 2
[0143] Example 2 2-((1R,5S,6s)-3-(2-(3,3-difluoro-2-methylazepin-1-yl)-6-(trifluoromethyl)pyrimidine-4 Preparation of -yl)-3-azabicyclo[3.1.0]hexane-6-yl)acetic acid (compound 2)
[0144]
[0145] 1) 2-((1R,5S,6s)-3-(2-(3,3-difluoro-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidine Preparation of -4-yl)-3-azabicyclo[3.1.0]hex-6-yl)methyl acetate
[0146]
[0147] 2-((1R,5S,6s)-3-(2-chloro-6-(trifluoromethyl)pyrimidin-4-yl)-3-azabicyclo[3.1.0]hex-6-yl) Methyl acetate (60mg, 0.18mmol) was dissolved in acetonitrile (5mL), diisopropylaminoethylamine (0.16ml, 0.90mmol) and 3,3-difluoro-2-methylazetidine hydrochloride were added (39mg, 0.27mmol), reacted at 90°C for 6 hours, diluted with ethyl acetate (30mL), washed with saturated brine (10mL×3), dried the organic phase over anhydrous sodium sulfate, spin-dried, and column chromatography (25% ethyl acetate ester / petroleum ether) to obtain the product (50 mg, yield 67%).
[0148] 2) 2-((1R,5S,6s)-3-(2-(3,3-difluoro-2-met...
experiment example 1
[0155] Experimental example 1 In vitro KHK kinase inhibitory activity test of the compound provided by the embodiment of the present invention
[0156] Test product: the compound synthesized by the embodiment of the present invention, its structural formula is shown in Table 1.
[0157] Experimental reagents:
[0158]
[0159]
[0160] Experimental consumables:
[0161]
[0162] laboratory apparatus:
[0163]
[0164] experimental method:
[0165] 1. Compound dilution
[0166] 1) Use DMSO to prepare the compound of the present invention and the positive control drug to 10 mM as the test stock solution.
[0167] 2) Dilute the stock solution of the compound of the present invention 10 times to 1 mM, then dilute the compound of the present invention 3 times to 11 concentrations, the highest concentration is 1 mM, dilute the stock solution of the positive control drug 100 times to 0.1 mM, then, 3 times Gradually dilute the positive control drug to 11 concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com