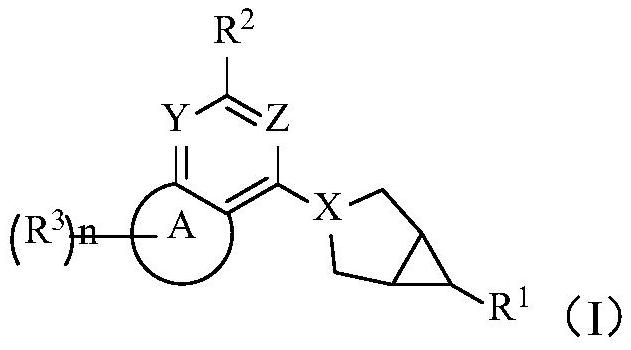
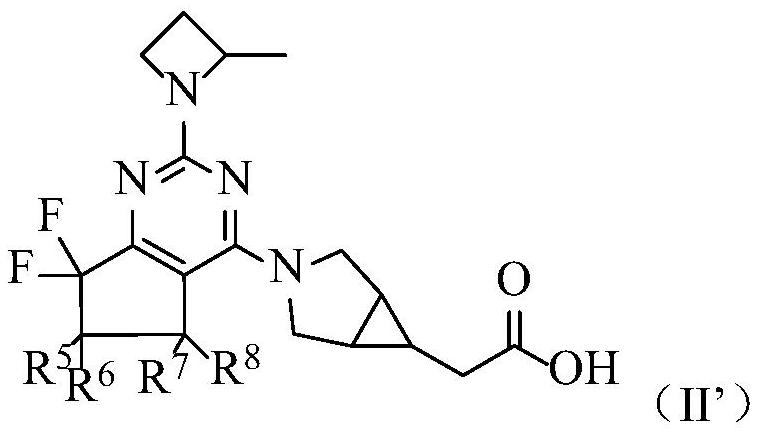
Deuterated hexanokinase inhibitor and application thereof
A deuterium substitution, compound technology, applied in the field of deuterated hexokinase inhibitors and their uses, can solve the problems of unmet clinical needs, endanger the lives of patients, etc., and achieve good pharmacokinetic properties, good safety, High bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
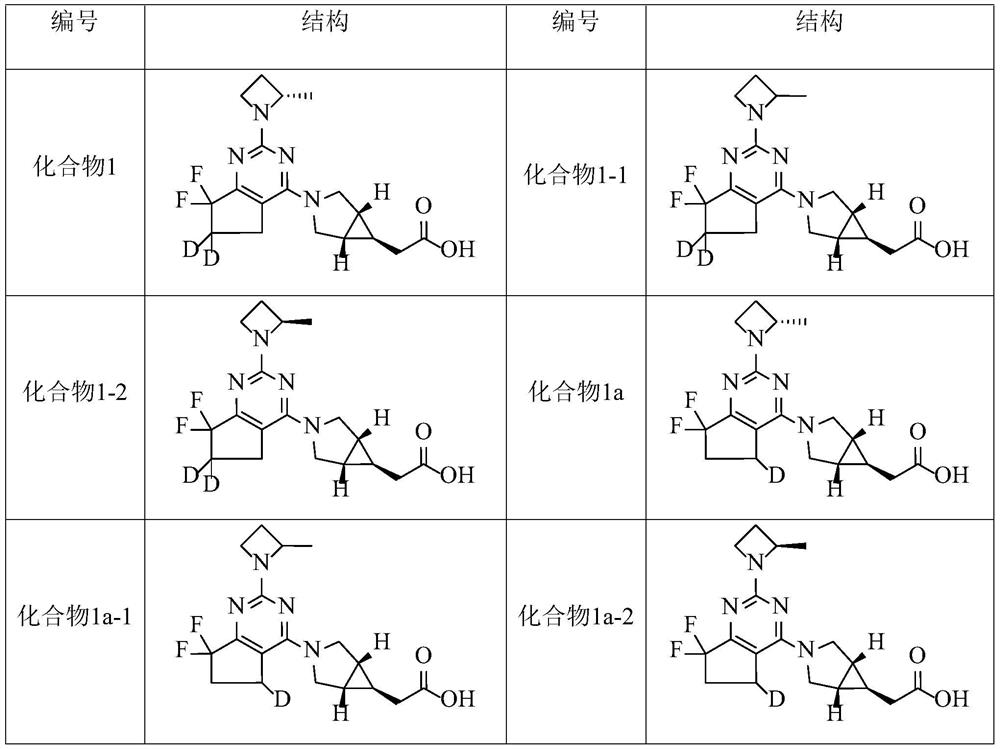
[0218] Example 1 2-((1R,5S,6R)-3-(7,7-difluoro-2-((S)-2-methylazetidin-1-yl)-6,7- Preparation of Dihydro-5H-cyclopenta[d]pyrimidin-4-yl-6,6-dideuterium)-3-azabicyclo[3.1.0]hexyl-6-yl)acetic acid (compound 1)
[0219] (1) Preparation of 1-ethyl 2,4-dimethyl 1-oxobutane-1,2,4-tricarboxylate
[0220]
[0221] Dissolve sodium ethoxide (6.1g, 90.0mmol) in THF (150mL), add diethyl oxalate (13.1g, 90.0mmol) until the solution is clear, then add dimethyl glutarate (14.4g, 90.0mmol), After the addition was complete, the reaction was carried out at 15°C for 18.5h. Concentrate the solvent, dilute with water, extract with methyl tert-butyl ether, adjust the pH of the aqueous phase to 1 with concentrated hydrochloric acid, extract with ethyl acetate, dry and concentrate the organic phase to obtain 8.9 g of the target compound, wash the methyl tert-butyl ether phase with hydrochloric acid, The organic phase was dried and concentrated to obtain 10 g of the target compound, and a total o...
Embodiment 2
[0254] Example 2 2-((1R,5S,6R)-3-(7,7-difluoro-2-((S)-2-methylazetidin-1-yl)-6,7- Preparation of dihydro-5H-cyclopenta[d]pyrimidin-4-yl-5-deuterium)-3-azabicyclo[3.1.0]hexyl-6-yl)acetic acid (compound 1a)
[0255] (1) Preparation of dimethyl glutarate-3-deuterium
[0256]
[0257] Add dimethyl glutaconate (6.08g, 38.5mmol) to 50mL of methanol, add 15mL of tetrahydrofuran, nickel chloride (2.4g, 18.5mmol), cool to 0°C, add sodium borodeuteride (2.2 g, 52 mmol), continue to stir for 0.5 h, filter with celite, concentrate, and purify on a silica gel column (petroleum ether: ethyl acetate = 5:1) to obtain 5.9 g of the target compound, yield: 95.2%.
[0258] (2) Preparation of 1-ethyl 2,4-dimethyl 1-oxobutane-1,2,4-tricarboxylate-3-deuterium
[0259]
[0260] Dissolve sodium ethoxide (2.3g, 33.8mmol) in THF (50mL), add diethyl oxalate (4.9g, 33.5mmol) until the solution is clear, then add dimethyl glutarate-3-deuterium (5.4g, 33.5mmol), after the addition was completed, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com