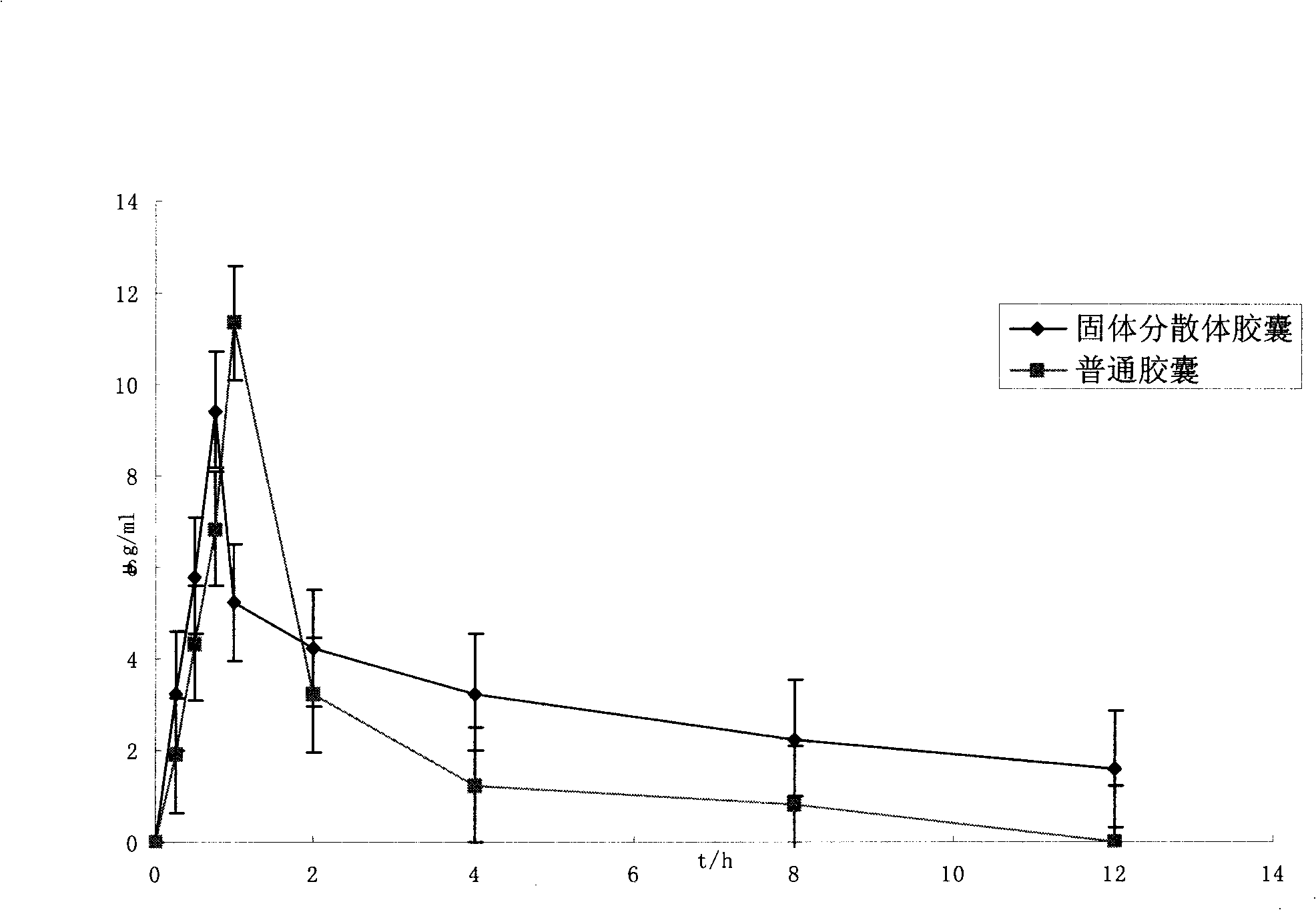
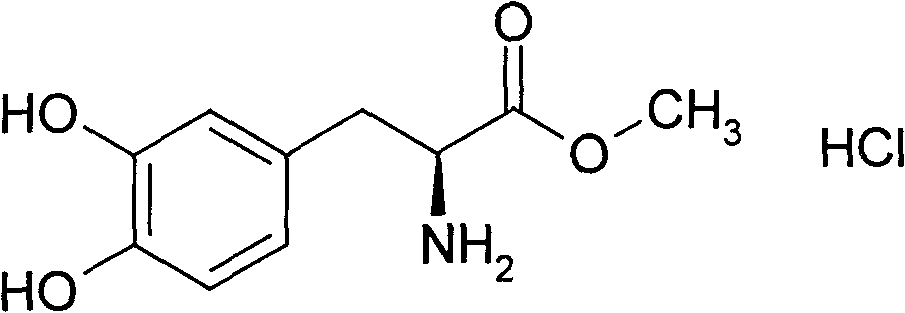
Preparation containing bendopa methyl ester and preparation method thereof
A technology of levodopa methyl ester and preparation, which is applied in the field of prescription and preparation of levodopa methyl ester solid dispersion, and can solve problems such as high drug concentration, fluctuation of blood drug concentration, and gastrointestinal irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Levodopa methyl ester 1
[0029] PEG6000 10
[0030] Lactose 0.2
[0032]
[0033] Take 1 part by weight of levodopa methyl ester, 10 parts by weight of PEG6000, and 0.2 part by weight of lactose, first pass PEG6000 through a 65-mesh sieve, heat to melt, and then gradually add levodopa methyl ester (passed 100-mesh sieve) and lactose (passed through a 100-mesh sieve), stir vigorously until completely melted, and set to cool to solidify. After curing, put it in a vacuum drying oven, take it out after embrittlement, pulverize it, and pass through an 80-mesh sieve to obtain a solid dispersion of levodopa methyl ester. Get this solid dispersion and magnesium stearate, mix uniformly, pack into capsules, and make capsules.
Embodiment 2
[0035] Levodopa methyl ester 1
[0036] PEG4000 2
[0037] Lactose 0.1
[0038] Microcrystalline Cellulose 2
[0039] starch 3
[0040] Sodium carboxymethyl starch 1
[0041] Magnesium stearate 0.06
[0042]
[0043] Take 1 part by weight of levodopa methyl ester, 2 parts by weight of PEG4000, and 0.1 part by weight of lactose, first pass PEG4000 through a 65 sieve, heat to melt, and then gradually add levodopa methyl ester in small amounts (over 100 mesh sieve) and lactose (passed through a 100 mesh sieve), stir vigorously until completely melted, and set to cool to solidify. After curing, put it in a vacuum drying oven, take it out after embrittlement, pulverize it, and pass through an 80-mesh sieve to obtain a solid dispersion of levodopa methyl ester. Get this solid dispersion and mix homogeneously with microcrystalline cellulose, starch, carboxymethyl starch sodium, with 5% povidone K 30 Granulated, dried, mixed evenly with magnesium ste...
Embodiment 3
[0045] Levodopa methyl ester 1
[0046] Poloxamer 188 6
[0047] Lactose 0.3
[0048] Sucrose 1
[0049] Magnesium stearate 0.15
[0050]
[0051] Get 1 part by weight of levodopa methyl ester, 6 parts by weight of Poloxamer (Poloxamer) 188, and 0.3 part by weight of lactose, first pass Poloxamer (Poloxamer) 188 through a 65-mesh sieve, heat to melt, and then Gradually add levodopa methyl ester (passed through a 100-mesh sieve) and lactose (passed through a 100-mesh sieve) in small amounts and several times, stir vigorously until completely melted, and set to cool to solidify. After curing, put it in a vacuum drying oven, take it out after embrittlement, pulverize it, and pass through an 80-mesh sieve to obtain a solid dispersion of levodopa methyl ester. Get this solid dispersion and mix evenly with 1 part by weight of sucrose, add 5% povidone K 30 Granulate, dry, and mix evenly with magnesium stearate to prepare granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com