Metformin hydrochloride osmotic pump controlled release tablet and preparation method thereof
A technology of metformin hydrochloride and controlled-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of decreased hardness, damage to the functionality of excipients, and unsuitable purified water. , to achieve the effects of drug release maintenance, improved release uniformity, and good release regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
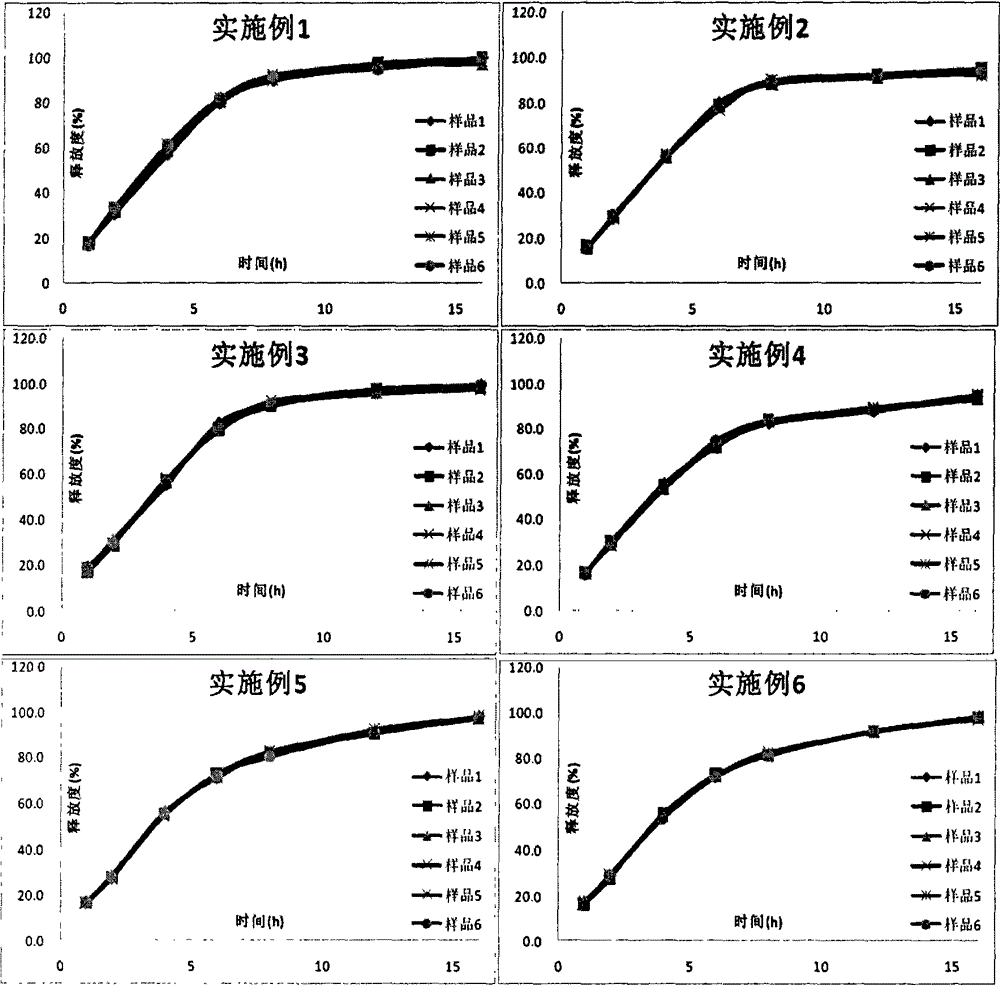
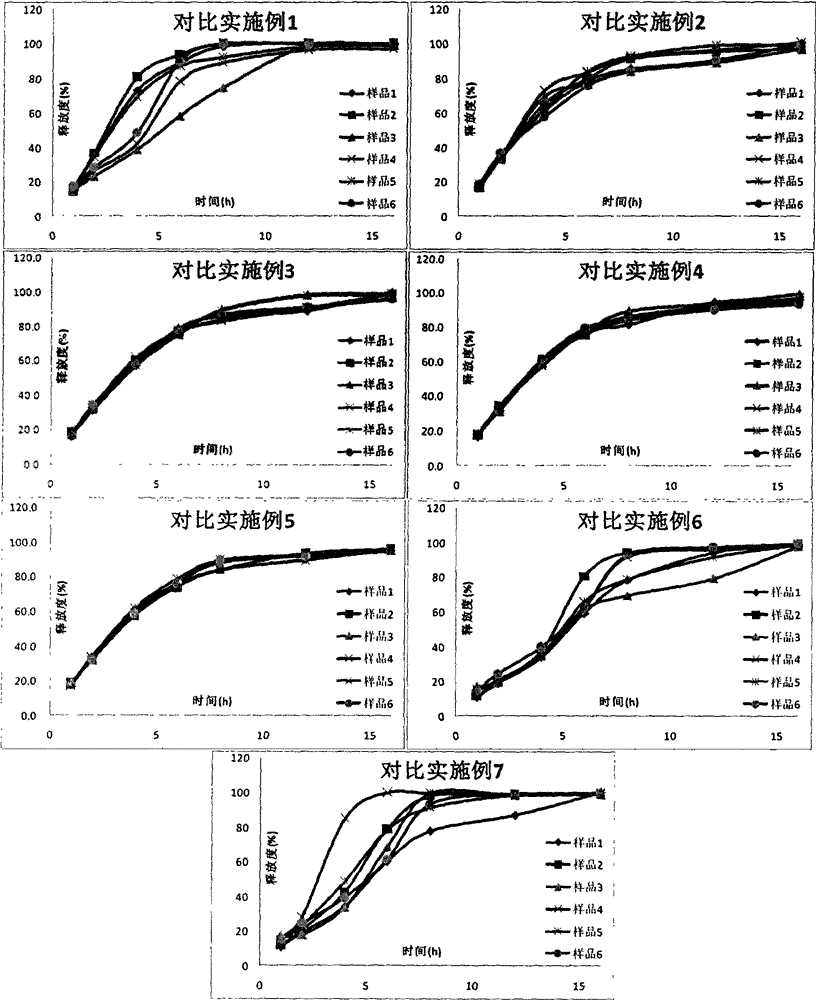
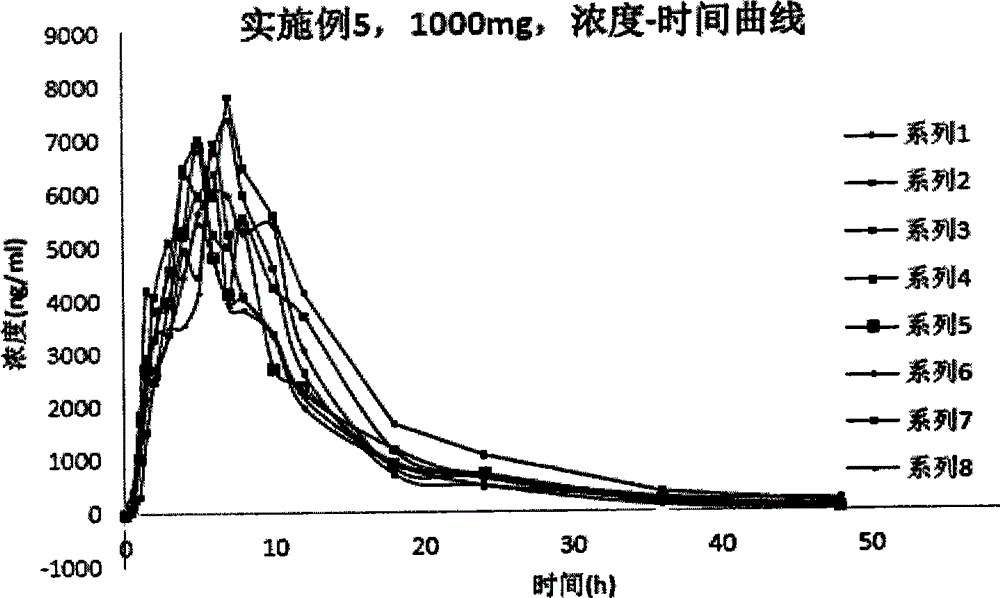
Examples
Embodiment 1
[0062] 1. Tablet core composition (mg / P)
[0063]
[0064] Granulation: powder metformin hydrochloride through a 100-mesh sieve, mix with other excipients except magnesium stearate evenly, use 95% ethanol to make soft material, wet granulate, dry below 40°C, control moisture at 2.0%
[0065] Below, 20 mesh sieve granules, add lubricant magnesium stearate and mix;
[0066] Tablet compression: use a shallow concave die to compress the tablets, set the rotary tablet press to compress the tablets, and control the hardness to 10-20kg / m2.
[0067] 2. Composition of the controlled-release coating layer
[0068]
[0069] 3. Composition of film coating
[0070]
[0071] Semi-permeable film coating: Take a certain amount of acetone, add cellulose acetate and PEG1500 to the acetone solution, stir to dissolve until a clear solution is obtained, and then use a high-efficiency coating pan to continuously coat the tablet core; the coating flow rate is about 100-150g / min, the tem...
Embodiment 2
[0076] Tablet core composition (mg / P)
[0077]
[0078] 1, semi-permeable membrane layer and film coating composition are the same as embodiment 1;
[0079] 2, production process is the same as embodiment 1;
[0080] 3. The weight gain of the coating is 4-6%.
Embodiment 3
[0082] Tablet core composition (mg / P)
[0083]
[0084] 1, semi-permeable membrane layer and film coating composition are the same as embodiment 1;
[0085] 2, production process is the same as embodiment 1;
[0086] 3. The weight gain of the coating is 4-6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com