Photosensitizer-chemotherapeutic drug 'photosensitizer-chemotherapeutic integration' micromolecule prodrug and establishment of self-assembled nanoparticle thereof
A technology of self-assembled nanoparticles and photochemical integration, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problem of crystallization of chemotherapeutic drugs and photosensitizers, and photosensitizer aggregation quenching , low drug loading, etc., to avoid aggregation quenching effect, easy surface modification, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
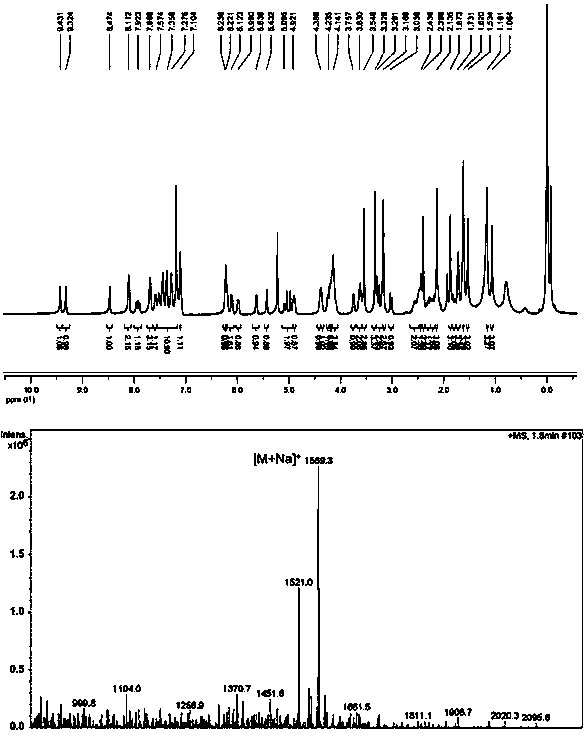
[0043] Example 1: Synthesis of pyropheophytin α-paclitaxel prodrug (PPa-S-PTX) linked by ROS sensitive bond
[0044] PPa, DMAP, HOBt and EDCI were dissolved in dichloromethane. The reaction solution was stirred at 0°C for 2 hours, then ethylene glycol was added and the reaction was continued for 48 hours at 37°C under nitrogen protection. The progress of the reaction was monitored by TLC. The target product was purified by preparative liquid chromatography to obtain PPa-EG. PTX, thiodiacetic anhydride and DMAP were dissolved in dichloromethane. The reaction solution was stirred at 25°C for 12 hours under nitrogen protection. The progress of the reaction was monitored by TLC. Purified using preparative liquid chromatography. The product was dissolved in dichloromethane with DMAP, HOBt and EDCI. The reaction solution was stirred at 0°C for 2 hours, then PPa-EG was added and the reaction was continued for 48 hours at 37°C under nitrogen protection. The target product was p...
Embodiment 2
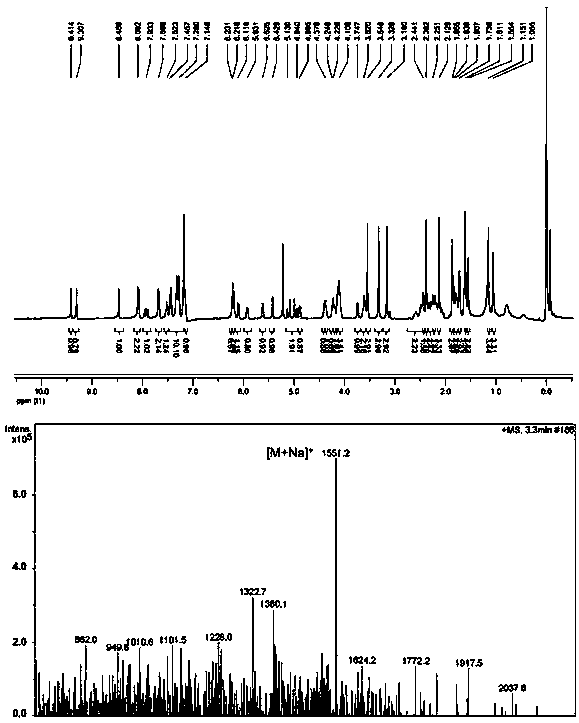
[0046] Embodiment 2: the synthesis of the porphyrin-paclitaxel prodrug (PPa-C-PTX) of non-ROS sensitive bond
[0047] PPa, DMAP, HOBt and EDCI were dissolved in dichloromethane. The reaction solution was stirred at 0°C for 2 hours, then ethylene glycol was added and the reaction was continued for 48 hours at 37°C under nitrogen protection. The progress of the reaction was monitored by TLC. The target product was purified by preparative liquid chromatography to obtain PPa-EG. PTX, glutaric anhydride and DMAP were dissolved in dichloromethane. The reaction solution was stirred at 25° C. for 12 hours under nitrogen protection. The progress of the reaction was monitored by TLC. Purified using preparative liquid chromatography. The product was dissolved in dichloromethane with DMAP, HOBt and EDCI. The reaction solution was stirred at 0°C for 2 hours, then PPa-EG was added and the reaction was continued for 48 hours at 37°C under nitrogen protection. The target product was pu...
Embodiment 3
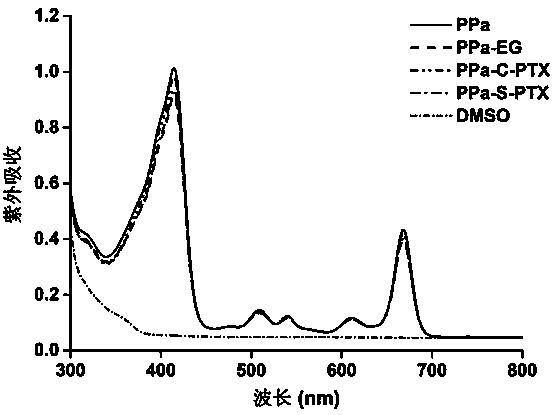
[0049] Embodiment 3: porphyrin-paclitaxel prodrug ultraviolet and fluorescence spectroscopic analysis
[0050] Dissolve PPa, PPa-EG, PPa-S-PTX and PPa-C-PTX in DMSO with the same concentration of PPa (2μM), and then use a multifunctional microplate reader to obtain their UV spectra and excitation wavelength at 415nm emission fluorescence spectrum. The result is as image 3 and Figure 4 As shown, no obvious changes were found in the peak positions and intensities of the ultraviolet spectrum and the fluorescence spectrum, indicating that the chemical modification has little effect on the optical properties of PPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com