Celecoxib nanosuspension and preparation method thereof
A nano-suspension, celecoxib technology, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of low bioavailability of celecoxib, large drug dosage, etc. The effect of increasing solubility and dissolution rate, simple formulation and process, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
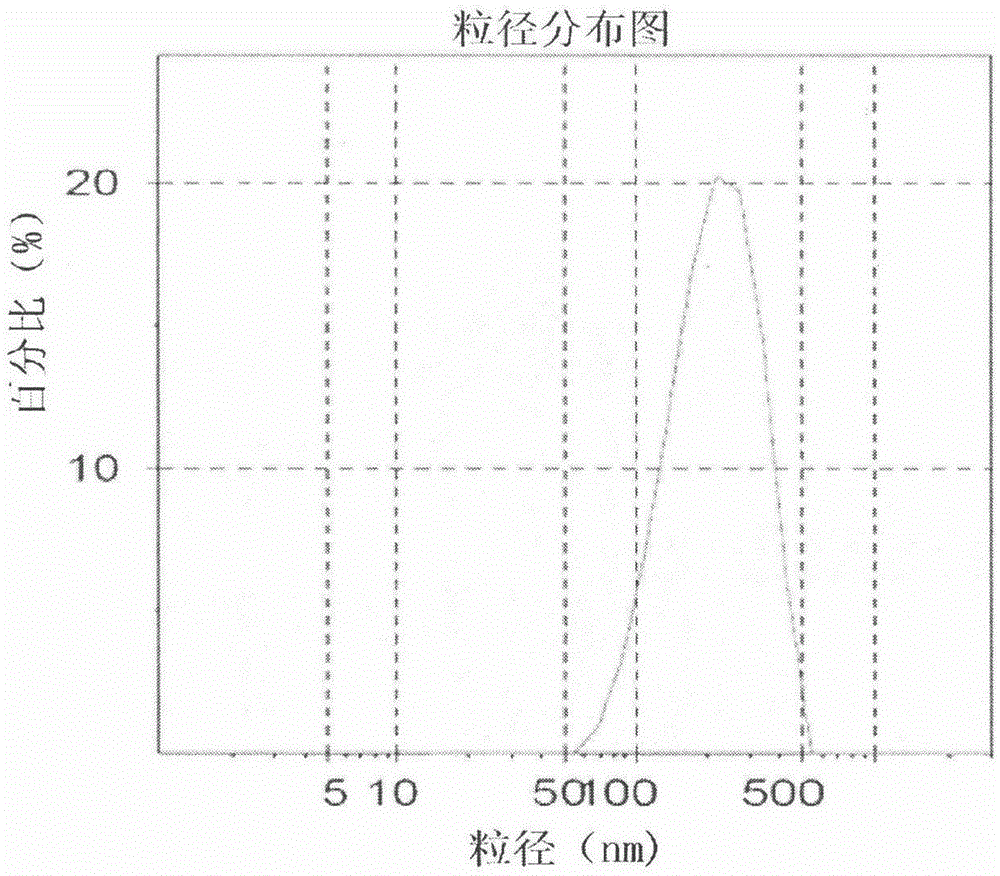
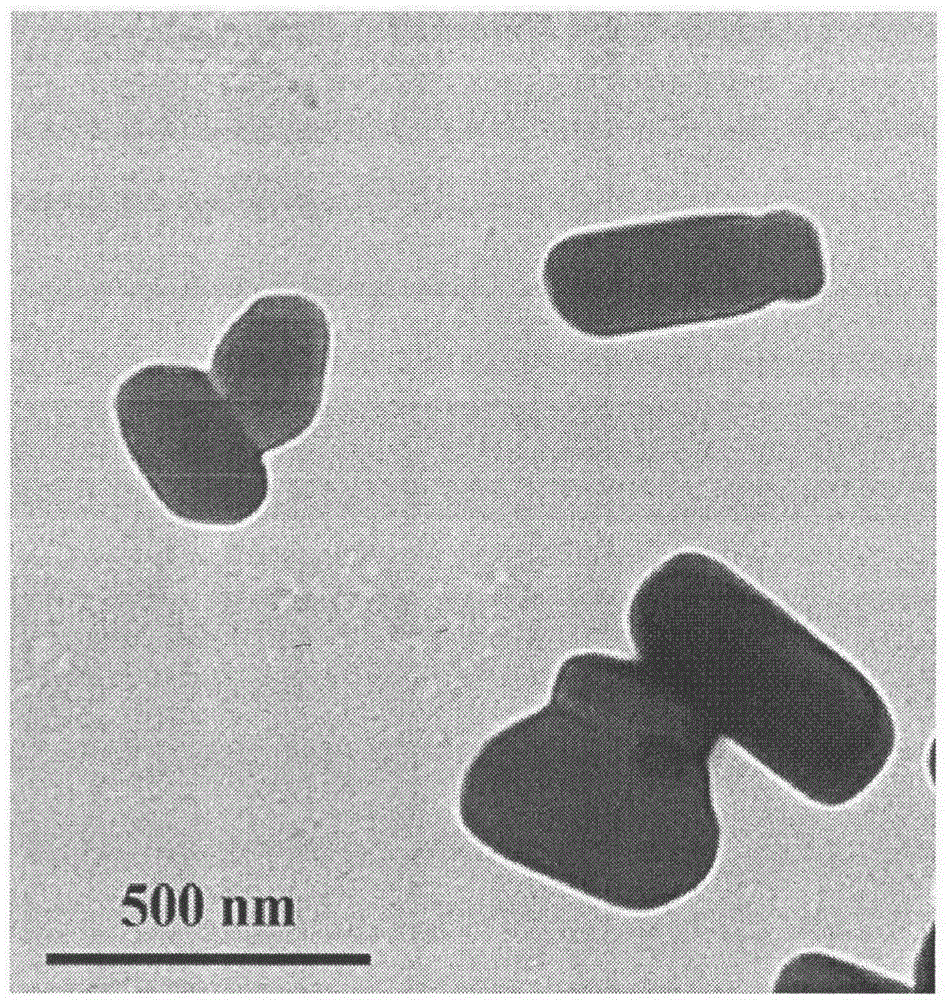
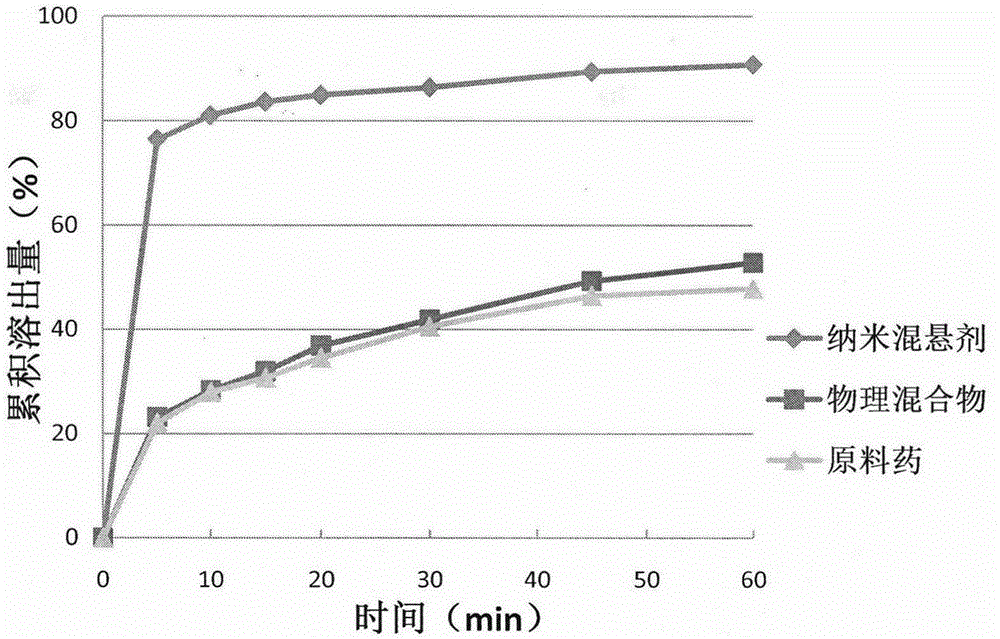
[0028] Weigh 0.25g of hypromellose, add 100mL of pure water to dissolve it completely, weigh 0.5g of celecoxib raw material, slowly pour it into the aqueous solution containing hypromellose under stirring, place in ultrasonic Sonicate in a washing machine for 5 minutes to disperse celecoxib in the aqueous stabilizer solution, and shear with a high-speed shear at 10,000 rpm for 5 minutes to obtain an initial suspension. The resulting primary suspension was poured into the feed port of a high-pressure homogenizer, and circulated 5 times at 200 bar and 15 times at 800 bar to obtain a celecoxib nanosuspension. The average particle size of the celecoxib nanosuspension prepared by this method is 382.2nm, the PI is 0.15, and the sedimentation ratio after standing for 48 hours is 95.7%.
Embodiment 2
[0030] Weigh 0.25g of poloxamer 188, add 100mL of pure water to dissolve it completely, weigh 0.5g of celecoxib raw material, slowly pour it into the aqueous solution containing poloxamer 188 under stirring, place in ultrasonic Sonicate in a washing machine for 5 minutes to disperse celecoxib in the aqueous stabilizer solution, and shear with a high-speed shear at 10,000 rpm for 5 minutes to obtain an initial suspension. The resulting primary suspension was poured into the feed port of a high-pressure homogenizer, and circulated 5 times at 200 bar and 15 times at 800 bar to obtain a celecoxib nanosuspension. The average particle size of the celecoxib nanosuspension prepared by this method is 251.3nm, the PI is 0.30, and the sedimentation ratio after standing for 48 hours is 92.5%.
Embodiment 3
[0032] Weigh 0.25g of polyvinylpyrrolidone K30, add 100mL of pure water to dissolve it completely, weigh 0.5g of celecoxib raw material, slowly pour it into the aqueous solution containing polyvinylpyrrolidone K30 under stirring, and place it in an ultrasonic cleaner Sonicate for 5 minutes to disperse celecoxib in the stabilizer aqueous solution, and shear at 10,000 rpm for 5 minutes with a high-speed shear to obtain an initial suspension. The resulting primary suspension was poured into the feed port of a high-pressure homogenizer, and circulated 5 times at 200 bar and 15 times at 800 bar to obtain a celecoxib nanosuspension. The average particle size of the celecoxib nanosuspension prepared by this method is 265.3nm, the PI is 0.29, and the sedimentation ratio after standing for 48 hours is 96.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com