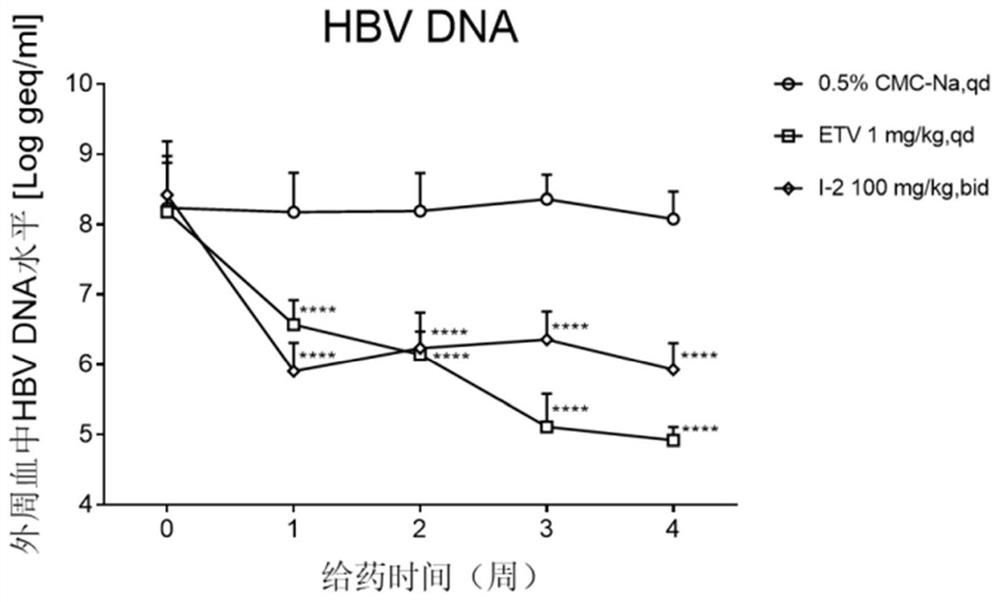
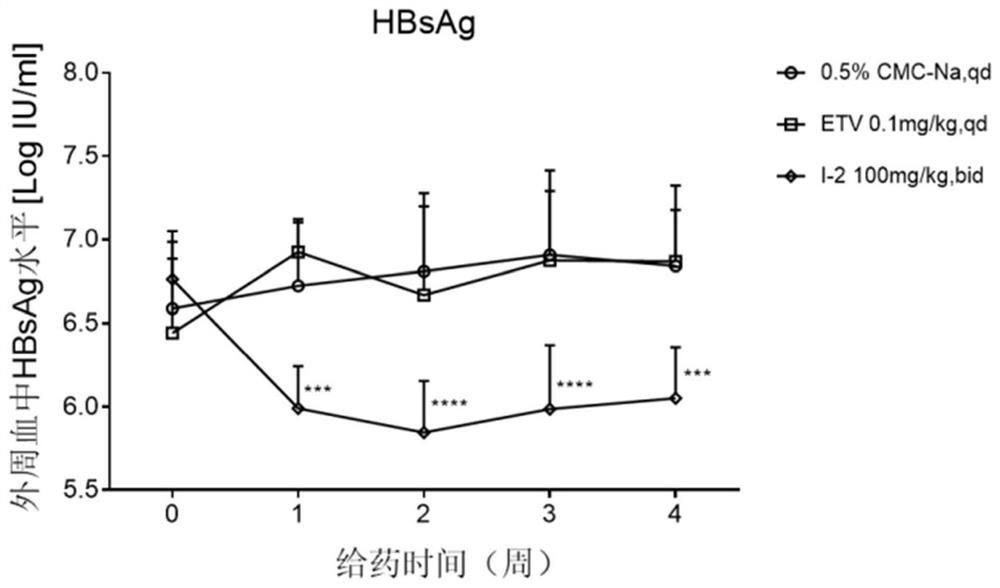
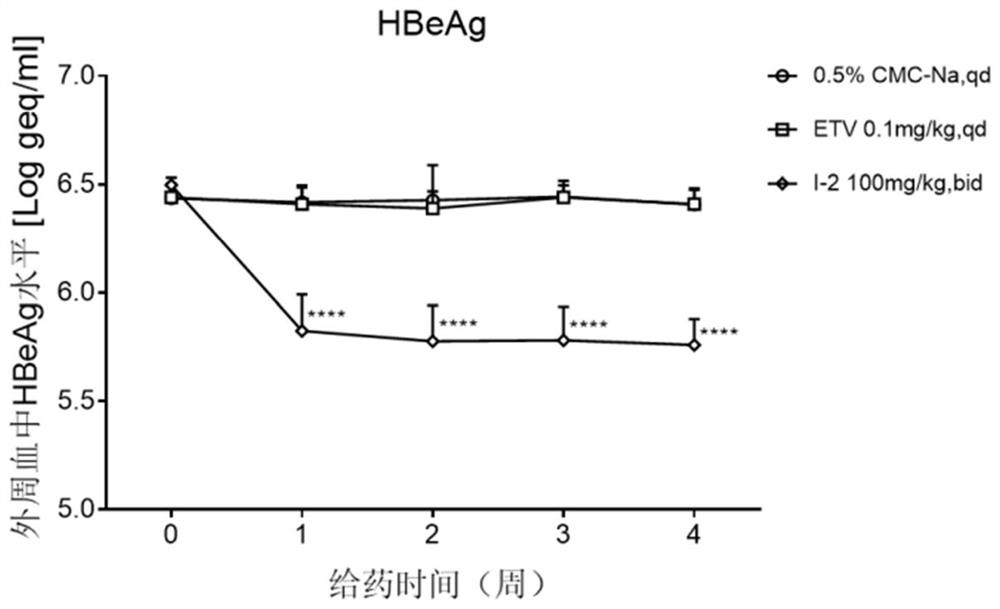
4-pyridine substituted phthalazinone compound as well as preparation method, pharmaceutical composition and application thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as many adverse reactions, poor interferon tolerance, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1: The synthetic route of compound 1 is as follows:
[0099]
[0100] Step (i):
[0101] 3,6-Dichlorophthalazine i-1 (10g, 50.3mmol) was added to acetic acid (150mL), and refluxed at 120°C for 6 hours. After the reaction was complete as monitored by TLC, the reaction was stopped and the reaction solution was cooled to room temperature and decompressed. Acetic acid was removed by rotary evaporation to obtain compound i-2 as a white solid.
[0102] Step (ii):
[0103] Compound i-2 (1.0g, 5.5mmol) was dissolved in DMF (70mL), and 4-cyanobenzyl chloride (1.26g, 6.6mmol) and Cs were added successively 2 CO 3 (2.1 g, 6.6 mmol). After the reaction system was stirred at 50° C. for 5 hours, the reaction was complete as monitored by TLC. Ethyl acetate (100 mL) and water (100 mL) were added to the reaction solution, the organic layer was separated, and the organic layer was washed successively with water (100 mL x 4) and saturated brine (100 mL). The organic ph...
Embodiment 2
[0131]
[0132] Step a:
[0133] Compound I-1 (85mg, 0.13mmol) was dissolved in dry tetrahydrofuran (5mL), added dropwise to 60% NaH (25mg, 64mmol) at 0°C, and stirred for 30 minutes. Add a solution of iodomethane (9.58uL, 0.15mmol) in tetrahydrofuran (2mL). After the addition is complete, the reaction solution is gradually raised to room temperature and stirred overnight. The reaction solution is poured into ice water, extracted with ethyl acetate (20mL×3), and the organic phase is anhydrous. It was dried over sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The residue was subjected to silica gel column chromatography to obtain compound I-33' (47mg, 55%) as a white powdery solid.
[0134] Step b:
[0135] Dissolve I-33' (40mg 0.059mmol) in 5mL of dichloromethane, add 0.5mL of trifluoroacetic acid, react in an oil bath at 40°C for 3h, add saturated sodium bicarbonate solution to adjust the pH value to neutral, extract with dichloromethane ...
Embodiment 3
[0139]
[0140]Step: Dissolve compound 1 (300mg, 0.65mmol) in tetrahydrofuran, under nitrogen protection, at -5°C, add a solution of tert-butylmagnesium bromide in tetrahydrofuran (1.35mmol, 1.35mL, c 1mol / L) successively, N -[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphoryl]-L-alanine isopropyl ester B (351mg, 0.775mmol) in tetrahydrofuran, react The temperature of the solution was gradually raised to 5°C, and the reaction was carried out for 17 hours. TLC monitors that there is no raw material, add water to quench the reaction, spin dry the solvent, add 100mL water, extract with ethyl acetate 3 times (50mL*3), wash with saturated brine, dry over anhydrous sodium sulfate, filter, and spin dry the solvent to obtain the crude product . The crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate 90 / 10-50 / 50) to obtain white solid compound I-36 (280 mg, 52%).
[0141]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com