Stearyl 11-deoxyglycyrrhetinate, derivative, cantharidin liposome, preparation method and application
A technology of cantharidin lipid and stearyl ester, applied in the field of preparation, 11-deoxyglycyrrhetinic acid stearyl ester, derivatives, cantharidin liposome field, can solve problems such as restricting wide application, pseudoaldosteronism, etc. , achieve the effects of inducing apoptosis, inhibiting tumor cell proliferation, and facilitating storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
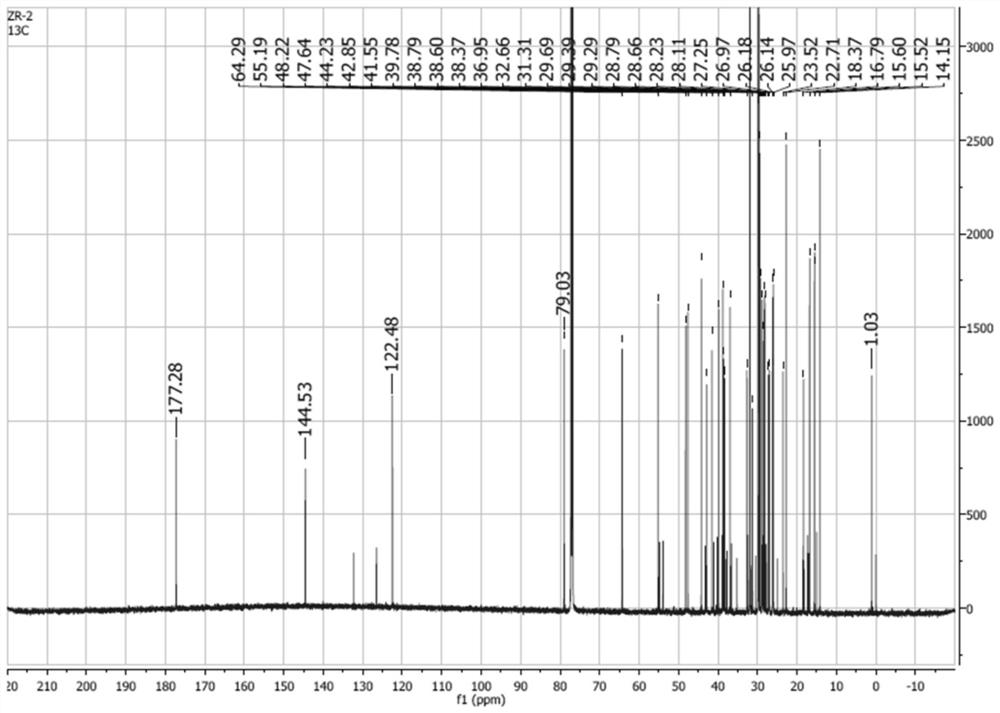
[0057] Synthesis of stearyl 11-deoxyglycyrrhetinate: take 0.45g HgCl 2 , add 15mL of hydrochloric acid solution with a concentration of 1.2mol / L, fully shake to dissolve, then add 4.5g of uniformly ground zinc powder, shake fully for 5min, remove the water phase, and wash twice with 5mL of dioxane to obtain , set aside; take 0.45g of the prepared zinc amalgam in a 50mL round bottom flask, add 0.72g of stearyl glycyrrhetinate and 13.5mL of dioxane to fully dissolve it, and use an ice-water bath to control the temperature at about 10°C. Add 1.5mL of hydrochloric acid with a concentration of 12mol / L within 30min, keep stirring at 10°C to continue the reaction for 5h after the addition is complete, TLC indicates the end point, pour the reaction solution into about 100mL of distilled water to precipitate a white precipitate, filter it with suction, dry it, and purify it. have to.
Embodiment 2
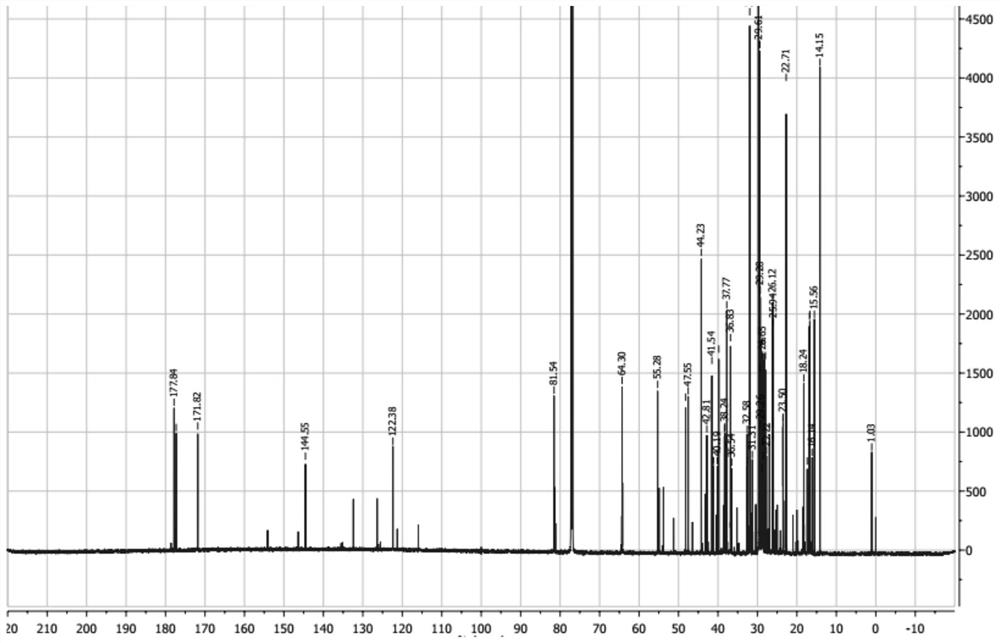
[0059] Synthesis of 11-stearyl deoxyglycyrrhetinate: take by weighing 0.70 g of stearyl deoxyglycyrrhetinate obtained in Example 1, 2.1 g of succinic anhydride and 0.01 g of DMAP (4-dimethylaminopyridine) in a circle Add 20mL of anhydrous double-distilled pyridine to the bottom flask, heat to reflux at 106°C, and check the reaction process by TLC. After the reaction, pour the reaction solution into 200mL of distilled water to form a brown precipitate, filter it, dry and purify it.
Embodiment 3
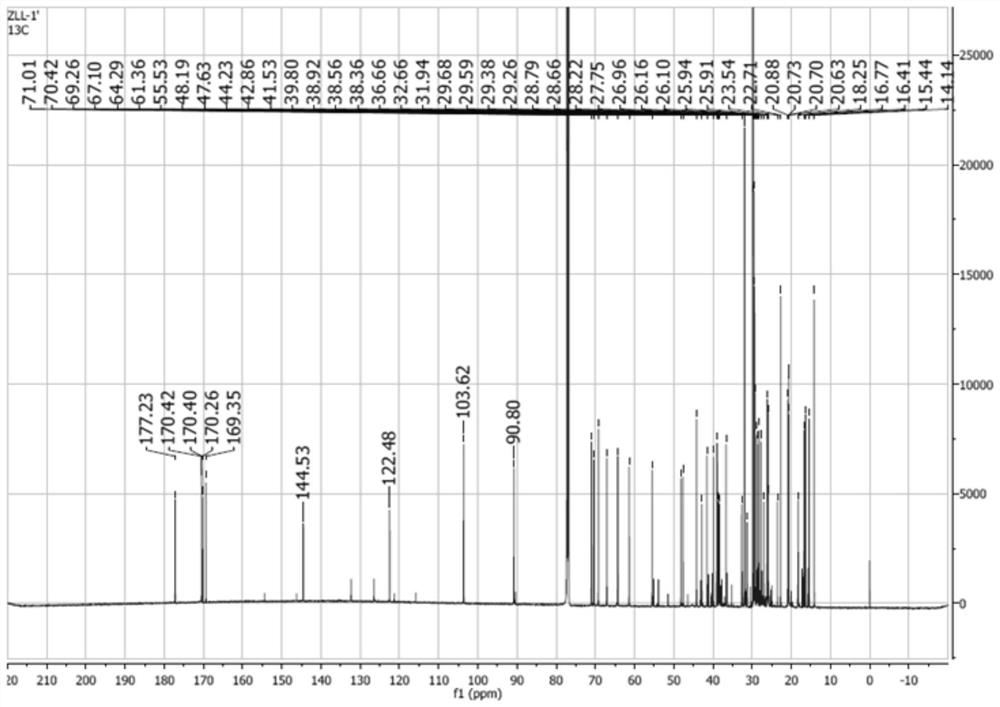
[0061] Synthesis of 11-stearyl deoxyglycyrrhetinate-3-O-galactoside: Weigh 4 g of stearyl deoxyglycyrrhetinate obtained in Example 1, 3.3 g of bromoacetyl galactoside, and 3.8 g of silver oxide , 8.7g of dried gypsum, 0.4g of iodine, and 20ml of chloroform were placed in a 50ml round-bottomed flask, mixed thoroughly, and magnetically stirred at 15°C for 5h. During the reaction period, the reaction process was detected by TLC every 1 h (developing agent: ethyl acetate-petroleum ether=1:5; thin-layer plate: silica gel G plate; chromogenic agent: iodine) until the end of the reaction. Filter the reaction solution, rinse the filter residue twice with 20ml of chloroform, collect the filtrate and evaporate to dryness on a water bath at 60°C, and purify to obtain 11-deoxyglycyrrhetinic acid stearyl-3-O-bromoacetylgalactoside ; Take 0.5 g of pure stearyl 11-deoxyglycyrrhetinate-3-O-galactoside, add 60 ml of 5 mg / ml methanol-sodium methoxide solution, and stir magnetically at 30°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com