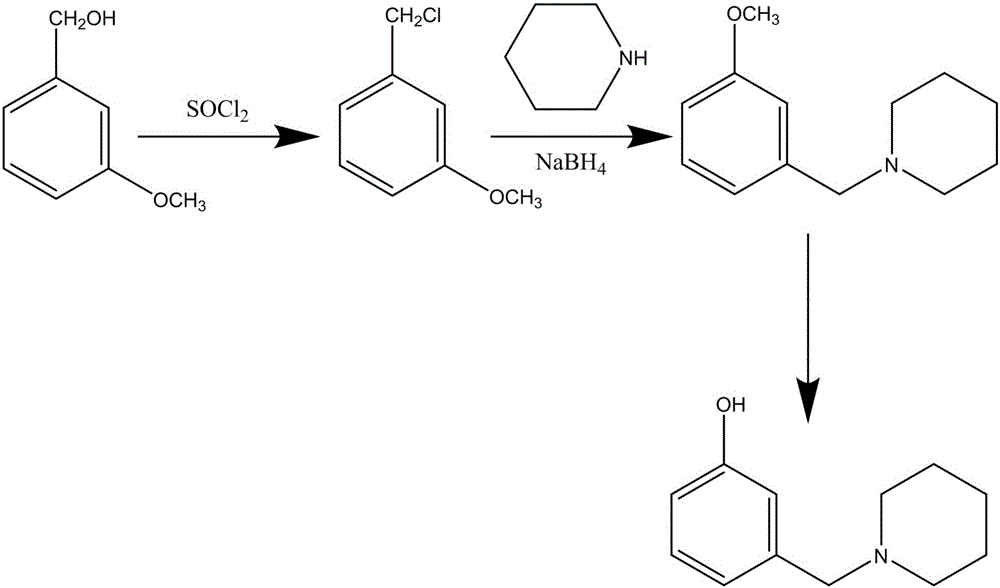
Method for synthesizing Roxatidine intermediate 3-(1-piperidinyl methyl) phenol
A technology of piperidinylmethyl and roxatidine, which is applied in the field of synthesis of roxatidine intermediate 3-phenol, can solve the problems of large amount of three wastes, dangerous operation, low yield, etc., and achieve less generation of three wastes , Reduce the preparation cost, the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Synthesis of m-methoxybenzyl chloride: 100g m-methoxybenzyl alcohol is added in 160ml thionyl chloride, the mixture is heated to reflux reaction for 1h, after the reaction is over, excessive thionyl chloride is recovered under reduced pressure to obtain 104g m-methoxybenzyl chloride oil, GC detection purity above 98%;
[0017] (2) Synthesis of 3-(1-piperidinylmethyl)anisole: add 100ml piperidine, 15g sodium borohydride and 100ml absolute ethanol to the oily substance obtained in the upward step, and the mixture is heated to reflux for 8 hours, and the reaction ends Recover ethanol under reduced pressure, then add 200ml of water to the residue, and add dilute hydrochloric acid to adjust the pH value to 3-4, take the water phase and add 100ml ethyl acetate for extraction, then take the resulting aqueous layer and adjust the pH value to 9 with ammonia water, and The precipitated oil was separated, and 131g of 3-(1-piperidylmethyl)anisole oil was obtained after washing ...
Embodiment 2
[0020] (1) Synthesis of m-methoxybenzyl chloride: 100g m-methoxybenzyl alcohol is added in 160ml thionyl chloride, the mixture is heated to reflux reaction for 1h, after the reaction is over, excessive thionyl chloride is recovered under reduced pressure to obtain 106g m-methoxybenzyl chloride oil, GC detection purity above 98%;
[0021] (2) Synthesis of 3-(1-piperidinylmethyl)anisole: add 100ml piperidine, 15g sodium borohydride and 100ml absolute ethanol to the oily substance obtained in the upward step, and the mixture is heated to reflux for 8 hours, and the reaction ends Recover ethanol under reduced pressure, then add 200ml of water to the residue, and add dilute hydrochloric acid to adjust the pH value to 3-4, take the water phase and add 100ml ethyl acetate for extraction, then take the resulting aqueous layer and adjust the pH value to 9 with ammonia water, and The precipitated oil was separated, and 135g of 3-(1-piperidylmethyl)anisole oil was obtained after washing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com