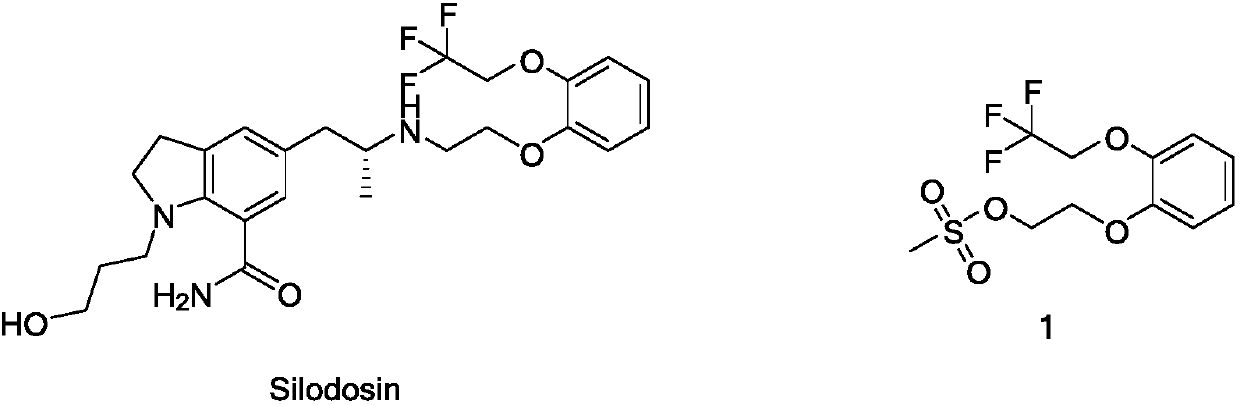
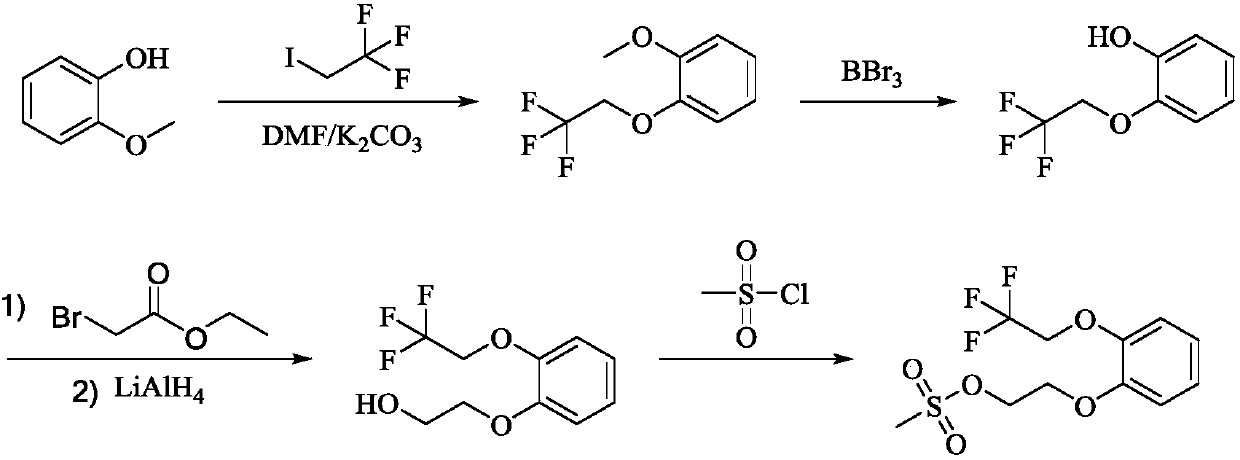
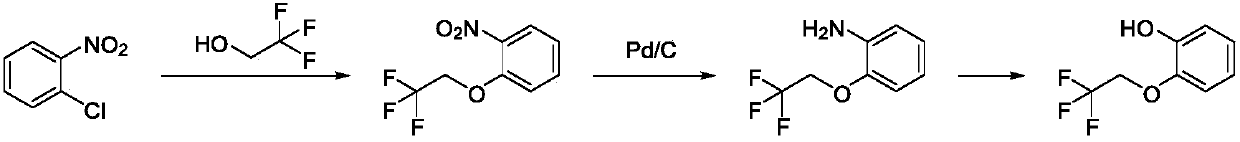
Preparation method of silodosin intermediate
A technology of silodosin and intermediates, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of difficulty in realizing industrialized production, expensive raw materials, and many side reactions in the system, and achieves broad prospects and industrial application value, and the conditions are uniform and controllable. , easy to operate and simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of preparation method of silodosin intermediate proposed by the present invention, the steps are as follows:
[0045] Synthesis of S1, 2-(2-hydroxyethoxy)benzaldehyde (formula Ⅲ)
[0046] In a 2000mL four-necked flask equipped with mechanical stirring, 200g (1.64mol, 1.0eq) of salicylaldehyde, 145g (1.64mol, 1.0eq) of ethylene carbonate, and 535g (1.64mol, 1.0eq) of cesium carbonate were added successively to participate in the reaction. ), dimethyl sulfoxide 600mL, N-methylpyrrolidone 600mL, under the protection of nitrogen, the temperature was raised to 130°C, and the reaction was incubated for 10 hours. After the sample HPLC raw materials were completely converted, the reaction system was cooled to room temperature and filtered. The filtrate was poured into 1000 mL of ice water, extracted with 500 mL*3 of ethyl acetate, the organic phases were combined, and dried over anhydrous sodium sulfate to obtain 200 g of light yellow oil with a yield of 73.3% and a puri...
Embodiment 2
[0054] A kind of preparation method of silodosin intermediate proposed by the present invention, the steps are as follows:
[0055] Synthesis of S1, 2-(2-hydroxyethoxy)benzaldehyde (formula Ⅲ)
[0056] In a 2000mL four-necked flask equipped with mechanical stirring, 200g (1.64mol, 1.0eq) of salicylaldehyde, 217g (2.46mol, 1.5eq) of ethylene carbonate, and 679g (4.92mol, 3.0eq) of potassium carbonate were added successively to participate in the reaction. ), N,N-dimethylacetamide 1000mL, heated up to 110°C under the protection of nitrogen, kept the reaction for 7 hours, and the raw materials were sampled by HPLC for complete conversion, the reaction system was cooled to room temperature, and filtered. The filtrate was poured into 1000 mL of ice water, extracted with 500 mL*3 of ethyl acetate, the organic phases were combined, and dried over anhydrous sodium sulfate to obtain 216 g of light yellow oil with a yield of 79.1% and a purity of 98.0%.
[0057] Synthesis of S2, 2-(2-h...
Embodiment 3
[0064] A kind of preparation method of silodosin intermediate proposed by the present invention, the steps are as follows:
[0065] Synthesis of S1, 2-(2-hydroxyethoxy)benzaldehyde (formula Ⅲ)
[0066] In a 2000mL four-necked flask equipped with mechanical stirring, 200g (1.64mol, 1.0eq) of salicylaldehyde, 188g (2.13mol, 1.3eq) of ethylene carbonate, and 680g (2.46mol, 3.0eq) of potassium carbonate were added successively. ), N,N-dimethylformamide 1000mL, heated up to 120°C under the protection of nitrogen, and kept the temperature for 9 hours to react. After sampling the HPLC raw material conversion was complete, the reaction system was cooled to room temperature and filtered. The filtrate was poured into 1000 mL of ice water, extracted with 500 mL*3 of ethyl acetate, the organic phases were combined, and dried over anhydrous sodium sulfate to obtain 214 g of light yellow oil with a yield of 78.4% and a purity of 98.2%.
[0067] Synthesis of S2, 2-(2-hydroxyethoxy)sodium ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com