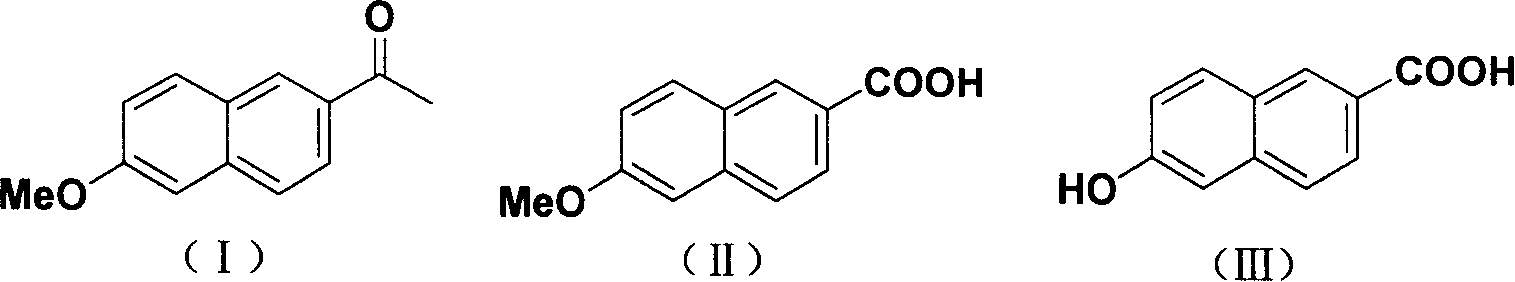
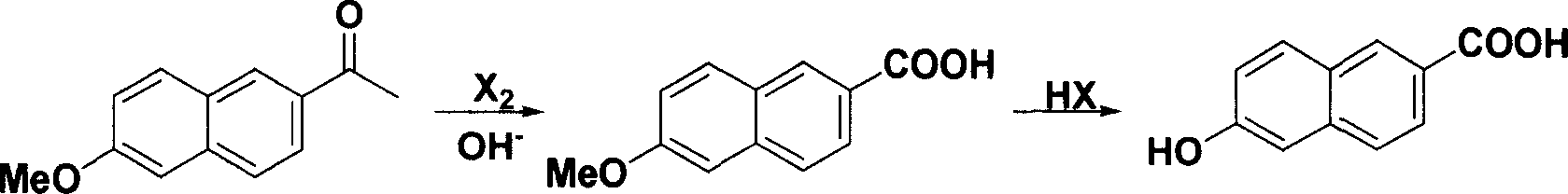
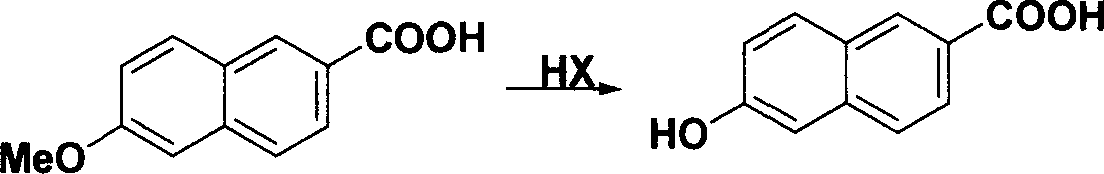Method for synthesizing 6-hydroxy-2-naphthoic acid
A synthetic method, naphthoic acid technology, applied in chemical instruments and methods, preparation of organic compounds, carboxylate preparation, etc., can solve the problems of rare raw materials, large investment in equipment, and only 37% reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 48.1g of sodium hydroxide (1.2mol) and 250mL of water into a 500mL three-necked flask, stir evenly under ice bath cooling, slowly add 15mL of bromine (0.6mol) dropwise to control the reaction temperature below 5°C, control the low temperature to continue Stir for 20min. Add 80mL dioxane, 27.0g sodium hydroxide (0.67mol), 20.1gI to the prepared sodium hypobromite solution. The temperature was raised to 75°C, and the reaction was refluxed for 2 hours. Stop the reaction, lower the temperature to below 10°C, and continue stirring for 30 minutes. Filter, add 100% water to the obtained solid, and add concentrated hydrochloric acid to adjust the pH to 3-4 while stirring. Heat to reflux, stir for more than 1 hour, cool to below 10°C, and maintain for about 0.5 hours. After filtration, the filter cake was washed with 100 g of water and dried to obtain 20 g of a colorless solid. The resulting solid was crystallized from ethanol 70 g / DMF 10 g. 2-Methoxy-6-naphthoic acid w...
Embodiment 2
[0027] Add 20.0g sodium hydroxide (1.2mol) and 200mL water to a 500mL three-necked flask, stir evenly under ice bath cooling, slowly add 12mL bromine (0.48mol) dropwise to control the reaction temperature below 5°C, control the low temperature to continue Stir for 20min. Add 80mL dioxane, 27.0g sodium hydroxide (0.67mol), and 20.1g I to the prepared sodium hypobromite solution. The temperature was raised to 75°C, and the reaction was refluxed for 2 hours. Stop the reaction, lower the temperature to below 10°C, and continue stirring for 30 minutes. Filter, add 100 mL of water to the obtained solid, and add concentrated hydrochloric acid to adjust the pH to 3-4 while stirring. Heat to reflux, stir for more than 1 hour, cool to below 10°C, and maintain for about 0.5 hours. After filtration, the filter cake was washed with 100 mL of water and dried to obtain 20 g of a colorless solid. The resulting solid was crystallized from ethanol 70 g / DMF 10 g. 2-Methoxy-6-naphthoic acid ...
Embodiment 3
[0029] Add 48.1g of sodium hydroxide (1.2mol) and 250mL of water into a 500mL three-necked flask, stir evenly under ice bath cooling, slowly add 15mL of bromine (0.6mol) dropwise to control the reaction temperature below 5°C, control the low temperature to continue Stir for 20min. Add 80mL dioxane, 27.0g sodium hydroxide (0.67mol), 20.1gI to the prepared sodium hypobromite solution. The temperature was raised to 50° C., and the reaction was kept for 10 hours. Stop the reaction, lower the temperature to below 10°C, and continue stirring for 30 minutes. Filter, add 100 mL of water to the obtained solid, and add concentrated hydrochloric acid to adjust the pH to 3-4 while stirring. Heat to reflux, stir for more than 1 hour, cool to below 10°C, and maintain for about 0.5 hours. After filtering, the filter cake was washed with 100 mL of water and dried to obtain 19 g of a colorless solid. The resulting solid was recrystallized from ethanol 70 g / DMF 10 g. 2-Methoxy-6-naphthoic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com