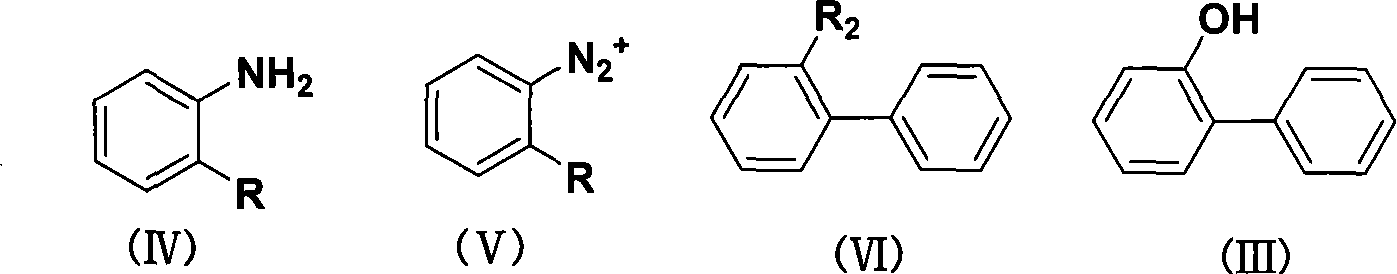
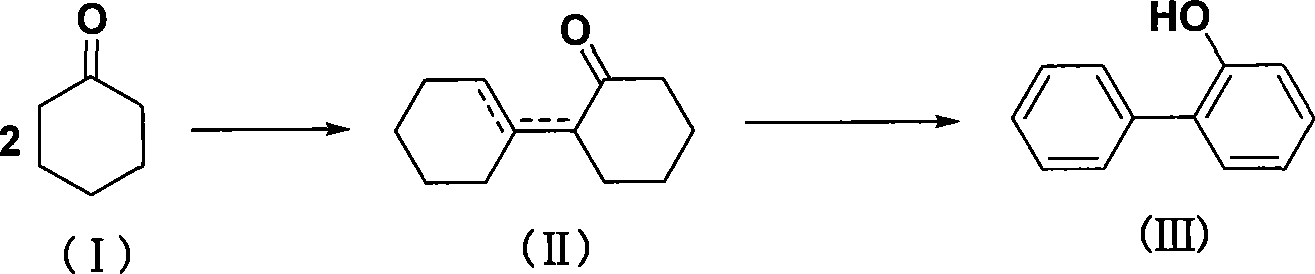
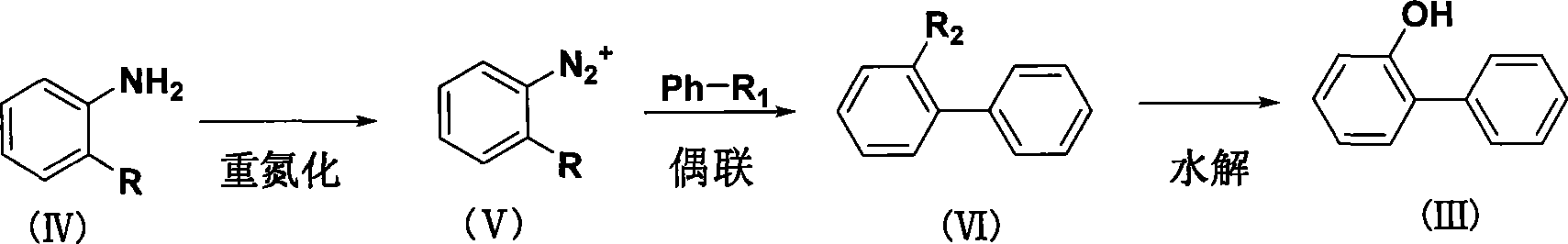
Method for synthesizing o-phenylphenol
A technology of o-phenylphenol and biphenyl, applied in the field of fine organic chemical industry, can solve the problems of high installation cost, limited output and high production cost, and achieve the effects of reducing risk factors, novel route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 6.4g of o-chloroaniline hydrochloride into a three-necked flask containing 30mL of benzene, add 3.0g of anhydrous magnesium sulfate and 0.1g of copper powder, and 0.05g of TEBA (benzyltriethylammonium chloride) at room temperature (25°C), Then add 3.3g of sodium nitrite, heat to 60°C and react for 24h, then add 1.0g of concentrated sulfuric acid, and continue to react for 12h. The reaction solution was filtered, the solid was washed twice with benzene, the filtrate was washed three times with water, and the benzene was recovered to obtain 5.6 g of o-chlorobiphenyl, with a yield of 76.16%.
Embodiment 2
[0030] Add 6.4g of o-chloroaniline hydrochloride into a three-neck flask containing 30mL of benzene, add 2.0g of anhydrous magnesium sulfate and 0.1g of copper powder, 0.05g of TEBA at room temperature (25°C), then add 3.3g of sodium nitrite, and heat to React at 60°C for 30 hours, then add 1.0 g of concentrated sulfuric acid, and continue the reaction for 15 hours. The reaction solution was filtered, the solid was washed twice with benzene, the filtrate was washed three times with water, and the benzene was recovered to obtain 5.3 g of o-chlorobiphenyl, with a yield of 72.11%.
Embodiment 3
[0032] Add 6.4g of o-chloroaniline hydrochloride into a three-necked flask containing 30mL of benzene, add 3.0g of anhydrous magnesium sulfate, 0.5g of copper powder, and 0.05g of TEBA at room temperature (25°C), then add 3.3g of sodium nitrite, and heat to React at 60°C for 20 hours, then add 1.0 g of concentrated sulfuric acid, and continue the reaction for 12 hours. The reaction solution was filtered, the solid was washed twice with benzene, the filtrate was washed three times with water, and the benzene was recovered to obtain 5.7 g of o-chlorobiphenyl, with a yield of 77.55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com