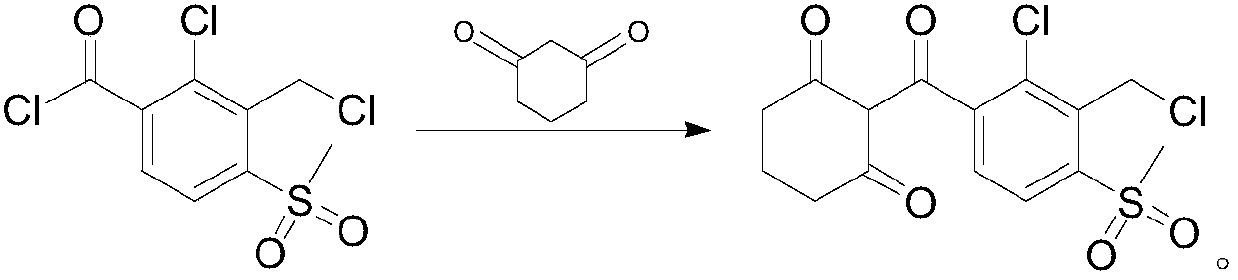
Preparation method of 2-(2-chloro-3-chloromethyl-4-methylsulfonyl benzoyl)-1,3-cyclohexanedione
A technology of methanesulfonylbenzoyl and methanesulfonylbenzoyl chloride is applied in the field of preparation of 2--1,3-cyclohexanedione, and can solve the problem of affecting the quality of the original drug of cyclosulfonone, without a synthesis method, and without Causes and other problems, and achieves the effect of being conducive to preparation and convenient operation, high selectivity, and guaranteed yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] This example prepares 2-(2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl)-1,3-cyclohexanedione through the following steps
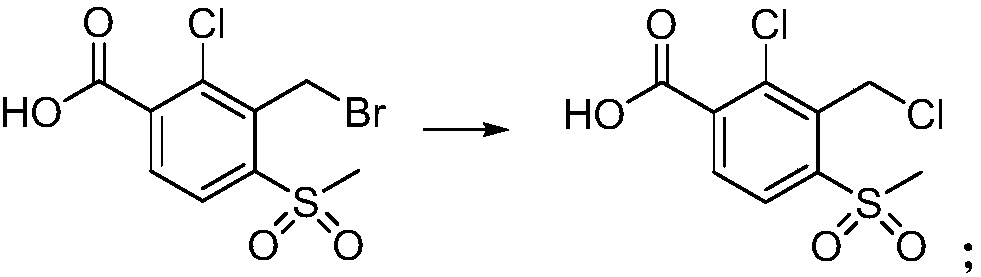
[0059] (1) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoic acid, the reaction formula is as follows:
[0060]
[0061] Add 2000mL 1,2-dichloroethane, 264.6g 2-chloro-3-bromomethyl-4-methylsulfonylbenzoic acid (99%, 0.8mol, prepared by referring to methods in WO2001007422 and US20040236146 ), 194.7g of concentrated hydrochloric acid (36%, 1.92mol) and 4g of sodium bromide, started stirring, and then heated up to 80°C for 10h. After the reaction was completed, 225.9 g of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoic acid was obtained through layering and precipitation, with an HPLC content of 97.8% and a reaction yield of 97.6%.
[0062] LC / MS, [M+1] + (%): 283 (100).
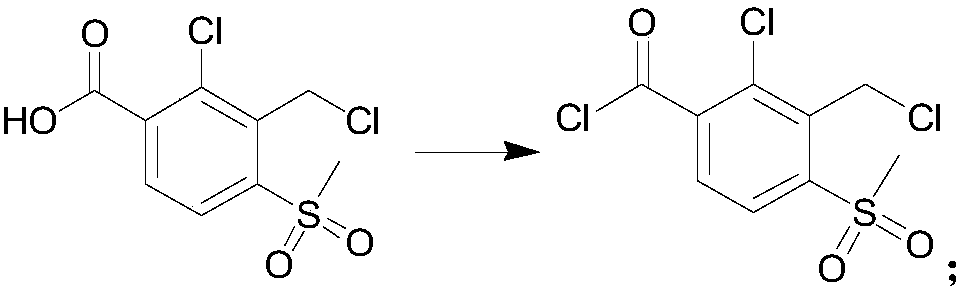
[0063] (2) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl chloride, the reaction formula is as follows:
[0064]
[0065] Add 786mL 1,2-dichlor...
Embodiment 2
[0072] This example prepares 2-(2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl)-1,3-cyclohexanedione through the following steps
[0073] (1) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoic acid, the reaction formula is as follows:
[0074]
[0075] Add 2000mL carbon tetrachloride, 264.6g 2-chloro-3-bromomethyl-4-methanesulfonylbenzoic acid (99%, 0.8mol, prepared with reference to methods in WO2001007422 and US20040236146), 162.25g Concentrated hydrochloric acid (36%, 1.6 mol) and 2.89 g of sodium bromide were started to stir, and then the temperature was raised to 90° C. for 8 h. After the reaction was completed, 223.1 g of 2-chloro-3-chloromethyl-4-methanesulfonylbenzoic acid was obtained through layering and precipitation. The HPLC content was 97.5%, and the reaction yield was 96.4%.
[0076] LC / MS, [M+1] + (%): 283 (100).
[0077] (2) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl chloride, the reaction formula is as follows:
[0078] ...
Embodiment 3
[0086] This example prepares 2-(2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl)-1,3-cyclohexanedione through the following steps
[0087] (1) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoic acid, the reaction formula is as follows:
[0088]
[0089] Add 2000mL chloroform, 264.6g 2-chloro-3-bromomethyl-4-methanesulfonylbenzoic acid (99%, 0.8mol, prepare with reference to the method in WO2001007422 and US20040236146), 243.4g concentrated hydrochloric acid ( 36%, 2.4mol) and 4.11g of sodium bromide, started stirring, and then heated to 70°C for 13h. After the reaction was completed, 217.6 g of 2-chloro-3-chloromethyl-4-methanesulfonylbenzoic acid was obtained through layering and precipitation, with an HPLC content of 96.8% and a reaction yield of 94.0%.
[0090] LC / MS, [M+1] + (%): 283 (100).
[0091] (2) Synthesis of 2-chloro-3-chloromethyl-4-methylsulfonylbenzoyl chloride, the reaction formula is as follows:
[0092]
[0093] Add 786mL toluene, 217.6g 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com