Preparation method of ketoprofen
A technology of ketoprofen and brominated benzophenone, which is applied in the field of medicine, can solve the problems of low yield, low yield, and affecting the total yield, etc., and achieve the effect of green process, easy price and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
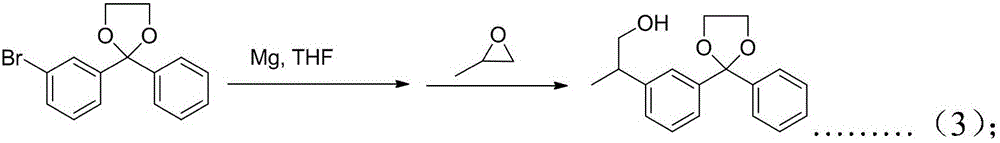
[0031] Summary is as follows: a kind of preparation method of ketoprofen, comprises the steps:
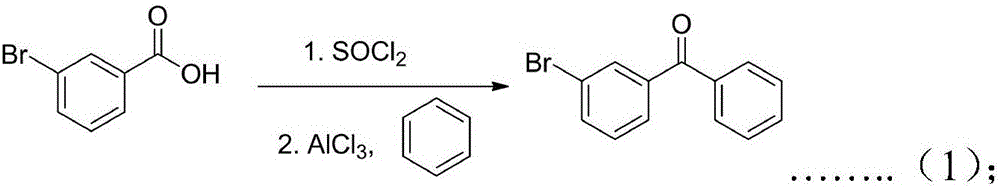
[0032] The synthesis of step 1,3-bromobenzophenone (1):
[0033] Using m-bromobenzoic acid and benzene as raw materials, synthesize (1) through acyl chloride and Friedel-Crafts acylation reaction, the catalyst used is aluminum trichloride, and the solvent is selected from dichloromethane, ethyl acetate, petroleum ether, ether, chloroform, Carbon disulfide or nitrobenzene:
[0034]
[0035] The specific operation of the step 1 is as follows: the mass ratio of the raw material for the synthesis of acid chloride to thionyl chloride is 1:1, the temperature range of the reaction is 50°C-70°C, and the reaction time of the acid chloride is 5-7h; For the Friedel-Crafts acylation reaction, the amount of catalyst used is a molar ratio of 1:(1.01-1.1), the temperature of the acid chloride is from room temperature to reflux, and the reaction time is 5-7h. After the reaction, the reaction s...
specific Embodiment 1
[0048] The preparation of specific embodiment 1.3-bromobenzophenone 1.
[0049]Dissolve 199g of m-bromobenzoic acid in 200g of thionyl chloride, and react at 70°C for 4h. After the reaction, recover the unreacted thionyl chloride under reduced pressure, and blow off the residual thionyl chloride in the reaction system by blowing with nitrogen. , cooled to 0 degrees in an ice bath, added 400g chloroform, 88g benzene, 140g anhydrous aluminum chloride to the reaction system, and reacted at room temperature for 6h. The mixture was separated, the chloroform layer was washed with water, dried over anhydrous sodium sulfate, the solvent was recovered under reduced pressure, and recrystallized from toluene to obtain 240 g of a white solid with a yield of 93%.
specific Embodiment 2
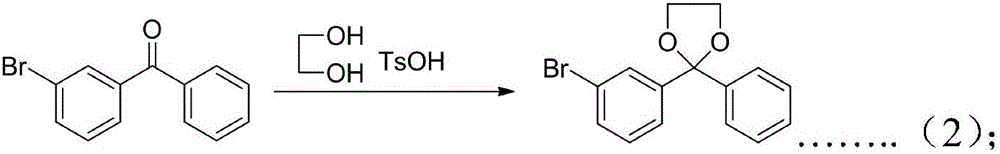
[0050] Specific Example 2. Synthesis 2 Preparation of 2-(3-bromophenyl)-2-phenyl-1,3-dioxolane.
[0051] 130g of 3-bromobenzophenone, 31g of ethylene glycol, and 1.5g of p-toluenesulfonic acid were dissolved in 200g of toluene and refluxed for 15h. During the reaction, the water in the water separator was removed, and toluene was added to the reaction system at the same time. Keep the liquid level of toluene basically constant. After the reaction, cool to room temperature, add 200 ml of 0.1 mol / L sodium hydroxide solution to the reaction system, separate the layers, wash the organic layer with water until neutral, dry over anhydrous sodium sulfate, filter, and recover the filtrate under reduced pressure, petroleum ether : Ethyl acetate = 10: 1 recrystallization to obtain 147 g of white solid, yield 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com