New synthesis process of sultopride hydrochloride
A technology of sultapride hydrochloride and hydrochloric acid, which is applied in the new synthesis field of anti-schizophrenia drug sutopride hydrochloride, can solve the problems of complicated post-processing, difficult recovery, and no practical value, and achieves stable yield and easy operation. , the effect of greatly improving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
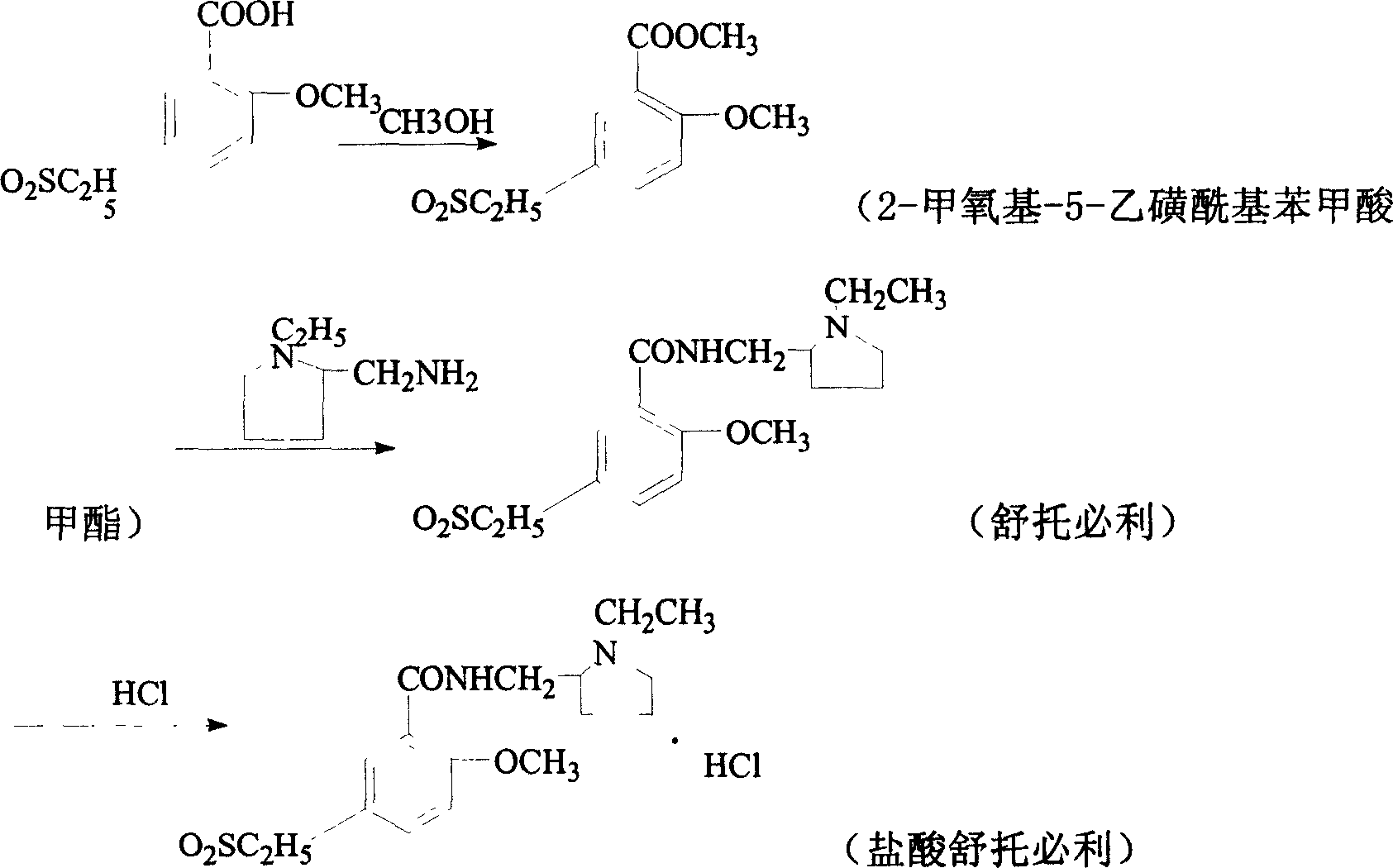
[0046] (1) Preparation of 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester
[0047] In a dry three-necked flask, first drop methanol (385ml), then slowly add concentrated sulfuric acid (38ml) under stirring, control the temperature to be less than 30°C, and finally add 2-methoxy-5-ethanesulfonylbenzoic acid ( 100g); after addition, stir and heat to reflux, react for 4hrs, place it under cooling, suction filter, wash with water until neutral, and obtain white crystal 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (91.5g), melting point 126.5 -128°C, yield 86.5%.
[0048] (2) preparation of sultopride hydrochloride
[0049] In a 500ml three-necked flask, put ethylene glycol (135ml) first, and then put N-ethyl-2-aminomethyltetrahydropyrrolidine (33.5ml) and 2-methoxy-5-ethanesulfonate successively under stirring. Methyl acylbenzoate (50g); after the injection, heat to 80±5°C and keep it warm for 10hrs. After the reaction, cool to room temperature, then acidify with hydroc...
Embodiment 2
[0051] (1) Preparation of 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester
[0052] In a dry there-necked flask, first drop methanol (500ml), then slowly add concentrated sulfuric acid (50ml) under stirring, control the temperature below 30°C, and finally add 2-methoxy-5-ethanesulfonylbenzoic acid ( 125g); after addition, stir and heat to reflux, react for 4hrs, cool and place, suction filter, wash with water to neutrality, and obtain white crystal 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (118.4g), melting point 126.5 -128°C, yield 89.6%.
[0053] (2) preparation of sultopride hydrochloride
[0054] In a 500ml three-necked bottle, put glycerin (55ml) first, then put N-ethyl-2-aminomethyltetrahydropyrrolidine (67ml) and 2-methoxy-5-ethanesulfonylbenzoic acid in turn under stirring Methyl ester (100g); after the injection, heat to 95±5°C and keep it warm for 6hrs. After the reaction, cool to room temperature, then acidify with hydrochloric acid to pH=1 to obtain of...
Embodiment 3
[0056] (1) Preparation of 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester
[0057]In a dry three-necked flask, first drop methanol (400ml), then slowly add concentrated sulfuric acid (40ml) under stirring, control the temperature below 30°C, and finally add 2-methoxy-5-ethanesulfonylbenzoic acid ( 100g); after adding, stir and heat to reflux, react for 4hrs, place it under cooling, filter with suction, wash with water until neutral, and obtain white crystal 2-methoxy-5-ethylsulfonylbenzoic acid methyl ester (93g), melting point 126.5- 128°C, yield 88%.
[0058] (2) preparation of sultopride hydrochloride
[0059] In a 500ml three-necked flask, put ethylene glycol (135ml) first, and then put N-ethyl-2-aminomethyltetrahydropyrrolidine (33.5ml) and 2-methoxy-5-ethanesulfonate successively under stirring. Methyl acylbenzoate (50g); after throwing in, heat to 90±5°C and keep it warm for 7hrs. After the reaction, cool to room temperature, then acidify with hydrochloric acid to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com