Preparation method of sofosbuvir key intermediate
An intermediate and key technology, which is applied in the field of preparation of key intermediates of sofosbuvir, can solve problems such as danger, high toxicity of reaction reagents, and harsh reaction conditions, and achieve the effects of simple equipment, novel routes, and short synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
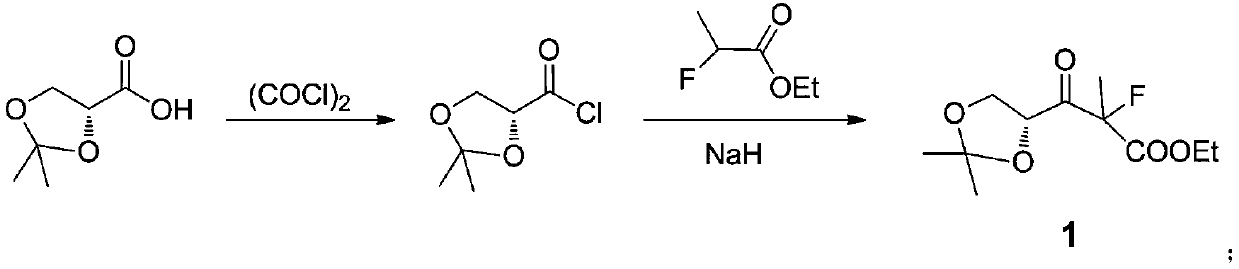
[0037] Example 1: Preparation of 3-((R)-2,2-dimethyl-1,3-dioxolanyl)-3-carbonyl-2-methyl-2-fluoropropionic acid ethyl ester (1 )
[0038] In a 500 milliliter round bottom flask, add 150 grams of β-acetone glyceride and 100 milliliters of dichloromethane, cool to 0° under an ice bath, add 70 grams of oxalyl chloride dropwise, during the dropping process, the temperature is controlled below 5 degrees, After 30 minutes of dripping, after the addition, keep warm for 3 hours. After the reaction, recover dichloromethane under reduced pressure, cool in an ice bath, precipitate oxalic acid, filter, and save the filtrate for future use. Take another No. 500 three-necked reaction flask, add 150 ml of tetrahydrofuran, cool to 0° in an ice bath, add 24 g of NaH, add 120 g of ethyl α-fluoropropionate in batches to the system, stir for 30 minutes, and cool the system to -10 °, dropwise add the β-glycerol acyl chloride acetone prepared above, during the dropping process, the system temperat...
Embodiment 2
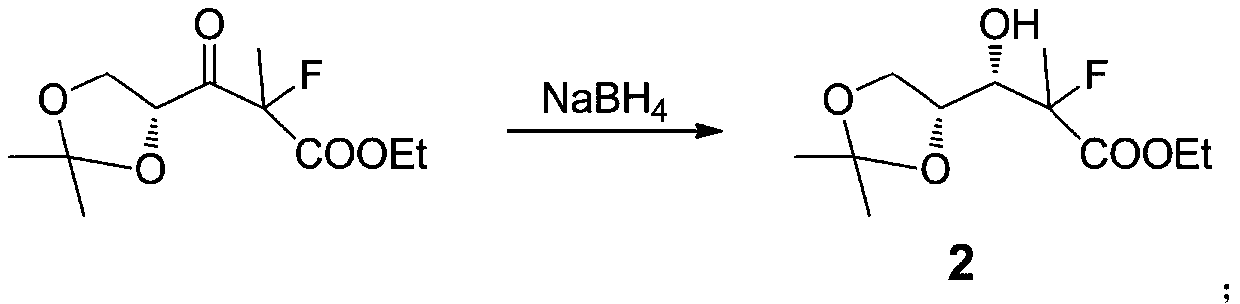
[0039] Example 2: Preparation of 3-((R)-2,2-dimethyl-1,3-dioxolanyl)-3-hydroxyl-2-methyl-2-fluoropropionic acid ethyl ester (2 )
[0040] Take a 500 ml round bottom flask, add 124 g of intermediate 1, 200 ml of ethanol, cool in an ice bath to 0 °, add 10 g of sodium borohydride in batches, during the addition process, control the temperature not to exceed 5 °, and complete the addition in 30 minutes. After the addition, the system continued to react for 2 hours. After the reaction was completed, 20 ml of dilute hydrochloric acid (1mol / L) was added to the reaction system, and the stirring was continued for 10 minutes. Extracted twice with ethyl ester, washed with water, combined organic phases, dried over anhydrous sodium sulfate, filtered, the filtrate recovered the solvent under reduced pressure, and the residue was distilled under reduced pressure to obtain 113 grams of light yellow liquid (5 mm Hg, collection temperature 90-94 °) , yield 91%.
Embodiment 3
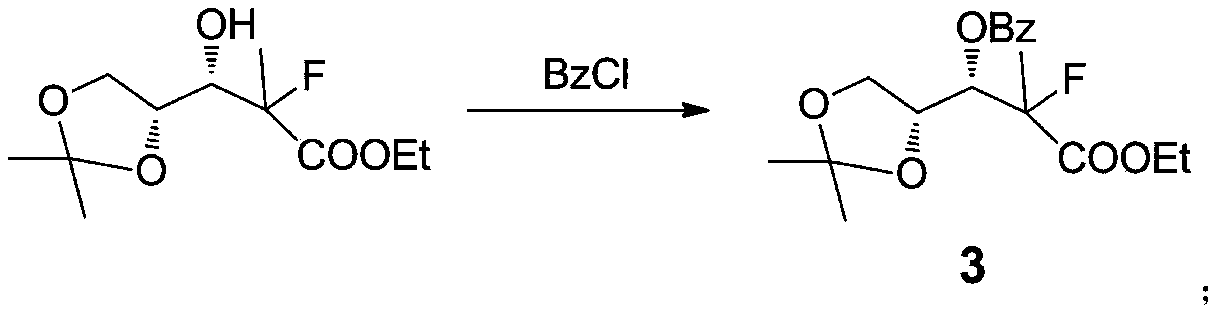
[0041] Example 3: Preparation of 3-((R)-2,2-dimethyl-1,3-dioxolanyl)-3-benzoyl-2-methyl-2-fluoropropionic acid ethyl ester (3)
[0042] Take a 500 milliliter three-necked reaction flask, install a constant pressure dropping funnel, nitrogen protection device, under nitrogen protection, add 62 grams of intermediate 3, 100 milliliters of dichloromethane, 30 grams of triethylamine to the system, and cool in an ice bath to 5 ° below, add 40 grams of Benjia acid chloride dropwise. During the dropping process, the temperature does not exceed 10 °. After the dropwise addition, the system is warmed up to 40 ° C for 1 hour. After the reaction, add 100 ml of water to the system, separate liquid, organic The phase was washed with water, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered from the filtrate under reduced pressure to obtain a yellow solid. Ethyl acetate:petroleum ether=1:1 was recrystallized to obtain 83.1 g of a light yellow solid, with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com