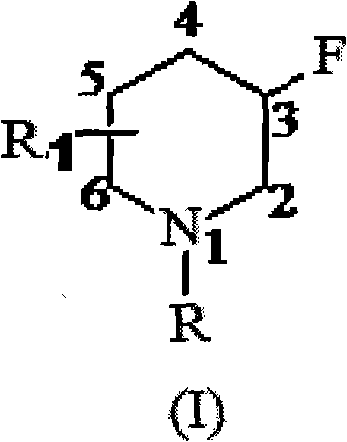
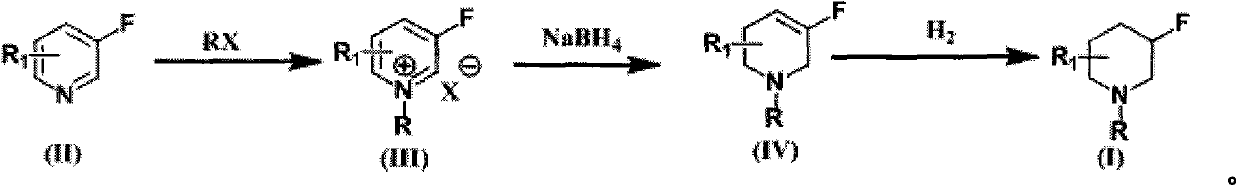3-droperidol derivative and preparation method thereof
A derivative, the technology of haloperidol, which is applied in the field of 3-fluoropiperidine derivatives and its preparation, can solve the problems of poor atom economy, difficulty in scaling up, and difficulty in post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Preparation of N-benzyl-3-fluoropyridinium quaternary ammonium salt
[0054]
[0055] 3-Fluoropyridine (291 g, 3 mol) was dissolved in 750 ml of toluene, and benzyl bromide (510 g, 3 mol) was added with stirring at room temperature. The mixture was heated to reflux for 3 hours. The temperature was lowered to room temperature and left overnight, the supernatant was poured out, and the lower solid was washed twice with petroleum ether, dried under vacuum, and directly proceeded to the next step.
Embodiment 2
[0056] Embodiment 2: Preparation of N-benzyl-3-fluoro-1,2,5,6 tetrahydropyridine
[0057]
[0058] Dissolve the N-benzyl 3-fluoropyridine quaternary ammonium salt in the previous step in 2000ml of methanol, add sodium borohydride in batches, and react for about 4 hours. After the reaction is complete as detected by TLC, add petroleum ether and extract three times (1000ml×l, 500ml×2 ). The petroleum ether phase was concentrated and directly distilled under reduced pressure to obtain 198 g of the product, with a two-step yield of 34.6%.
[0059] 1H NMR (300MHz, CDCl 3 )δ7.29~7.37(m, 5H); 5.24~5.33(dt, 1H); 3.66(s, 2H); 3.06~3.08(m, 2H); 2.56~2.59(m, 2H); m, 2H). MS-ESI: theoretical value.191; actual value: 192 (M+1) + .
Embodiment 3
[0060] Embodiment 3: Preparation of 3-fluoropiperidine
[0061]
[0062] Dissolve N-benzyl-3-fluoro-1,2,5,6-tetrahydropyridine (100g, 0.523mol) in 1L methanol, slowly add 10% Pd / C (10g), room temperature, under 1atm hydrogen After hydrogenation for 3 days, Pd / C was removed by filtration, the filtrate was adjusted to pH=1 with 10% hydrochloric acid, the aqueous phase was separated, concentrated to dryness, and recrystallized with tetrahydrofuran / methanol=10:1 to obtain 58 g of white solid with a yield of 75%.
[0063] 1HNMR (300MHz, DMSO-d6) δ9.30~9.41(br, 2H); 4.93(m, J=46Hz, 1H); 3.07~3.22(m, 2H); 2.84~3.01(m, 2H); 1.71~ 1.87 (m, 2H); 1.49-1.53 (m, 2H). MS-ESI: theoretical value.103; actual value: 104(M+1) + .
[0064] 3-Fluoropiperidine hydrochloride (10 g) was dissolved in water (10 ml), and the temperature was lowered to 0°C. Adjust pH=10 with sodium hydroxide, extract with dichloromethane (100ml*3), dry the combined organic phase over anhydrous sodium sulfate, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com