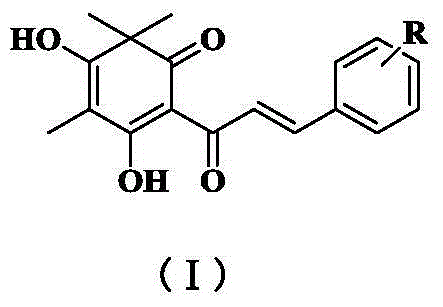
Quinoid chalcone compound with methyl group at A ring, and preparation method and anti-inflammatory activity thereof
A technology of trihydroxychalcones and compounds, applied in the field of medicine, can solve problems such as easy destruction, unstable chemical properties, and limited development and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthetic method of compound 1, comprises the following steps:
[0061] (1) Synthesis of 2-hydroxy-4,6-dimethoxymethoxyacetophenone
[0062] Add 1g of 2,4,6-trihydroxyacetophenone to anhydrous acetone, stir to dissolve, take 6.1g of potassium carbonate, add to acetone; after 15 minutes, take 1.13ml of chloromethyl methyl ether, add to acetone middle. After stirring and reacting for 5 hours, filter and dry the filtrate, and finally use a mixed solvent of petroleum ether / ethyl acetate with a volume ratio of 10:1, and perform silica gel column chromatography to obtain: 2.4g yellow oily solid 2-hydroxy-4,6-dimethyl Oxymethoxyacetophenone, productive rate is 79%; This process takes place following reaction:
[0063]
[0064] The structural characterization data of the product are: 1 HNMR (acetone-d 6 ,300MHz)δ2.643(3H,s,CH 3 ),3.443(3H,s,OCH 3 ),3.524(3H,s,OCH 3 ),5.242(2H,s,OCH 2 O),5.356(2H,s,OCH 2 O),6.195(1H,d,J=2.1Hz,H-3),6.292(1H,d,J=2.1Hz,H-5),13.738(1...
Embodiment 2
[0078] The synthetic method of compound 2, comprises the following steps:
[0079] (1) Synthesis of 2-hydroxy-4,6-dimethoxymethoxyacetophenone
[0080] Add 1g of 2,4,6-trihydroxyacetophenone to anhydrous acetone, stir to dissolve, take 6.1g of potassium carbonate, add to acetone; after 15 minutes, take 1.13ml of chloromethyl methyl ether, add to acetone middle. After stirring and reacting for 5 hours, filter and dry the filtrate, and finally use a mixed solvent of petroleum ether / ethyl acetate with a volume ratio of 10:1, and perform silica gel column chromatography to obtain: 2.4g yellow oil 2-hydroxy-4,6-dimethyl Oxymethoxyacetophenone, productive rate is 79%; This process takes place following reaction:
[0081]
[0082] The structural characterization data of the product are: 1 HNMR (acetone-d 6 ,300MHz)δ2.643(3H,s,CH 3 ),3.443(3H,s,OCH 3 ),3.524(3H,s,OCH 3 ),5.242(2H,s,OCH 2 O),5.356(2H,s,OCH 2 O),6.195(1H,d,J=2.1Hz,H-3),6.292(1H,d,J=2.1Hz,H-5),13.738(1H,s,OH)...
Embodiment 3
[0096] The synthetic method of compound 3 comprises the following steps:
[0097] (1) Synthesis of 2-hydroxy-4,6-dimethoxymethoxyacetophenone
[0098] Add 1g of 2,4,6-trihydroxyacetophenone to anhydrous acetone, stir to dissolve, take 6.1g of potassium carbonate, add to acetone; after 15 minutes, take 1.13ml of chloromethyl methyl ether, add to acetone middle. After stirring and reacting for 5 hours, filter and dry the filtrate, and finally use a mixed solvent of petroleum ether / ethyl acetate with a volume ratio of 10:1, and perform silica gel column chromatography to obtain: 2.4g yellow oil 2-hydroxy-4,6-dimethyl Oxymethoxyacetophenone, productive rate is 79%; This process takes place following reaction:
[0099]
[0100] The structural characterization data of the product are: 1 HNMR (acetone-d 6 ,300MHz)δ2.643(3H,s,CH 3 ),3.443(3H,s,OCH 3 ),3.524(3H,s,OCH 3 ),5.242(2H,s,OCH 2 O),5.356(2H,s,OCH 2 O),6.195(1H,d,J=2.1Hz,H-3),6.292(1H,d,J=2.1Hz,H-5),13.738(1H,s,OH)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com