Patents
Literature
52 results about "2-Oxazolidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
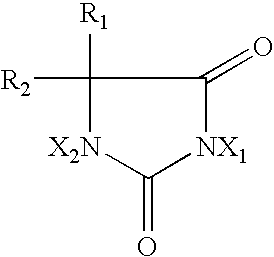
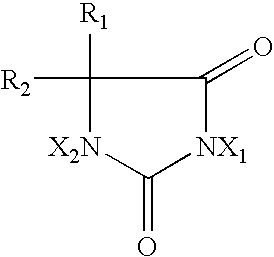
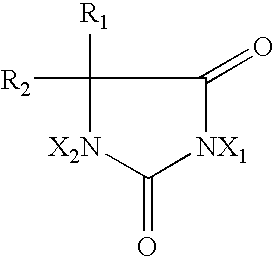
2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.
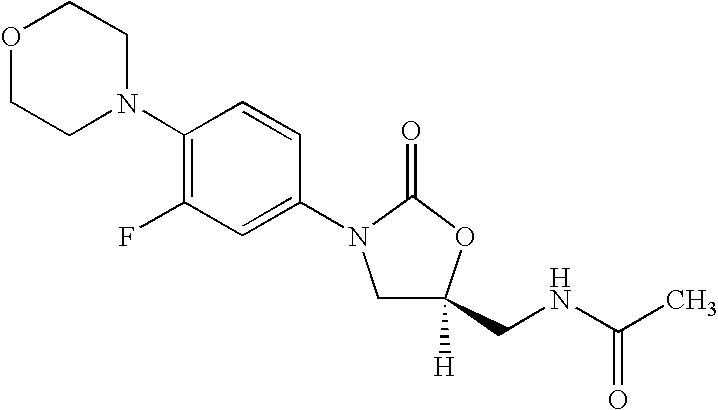
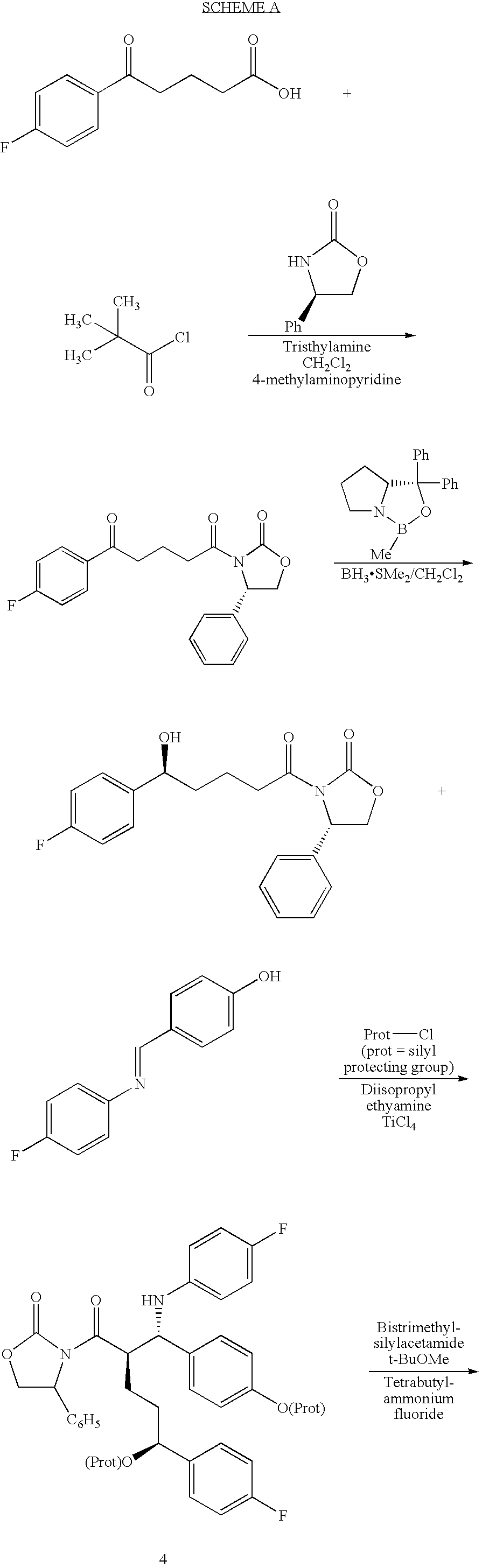
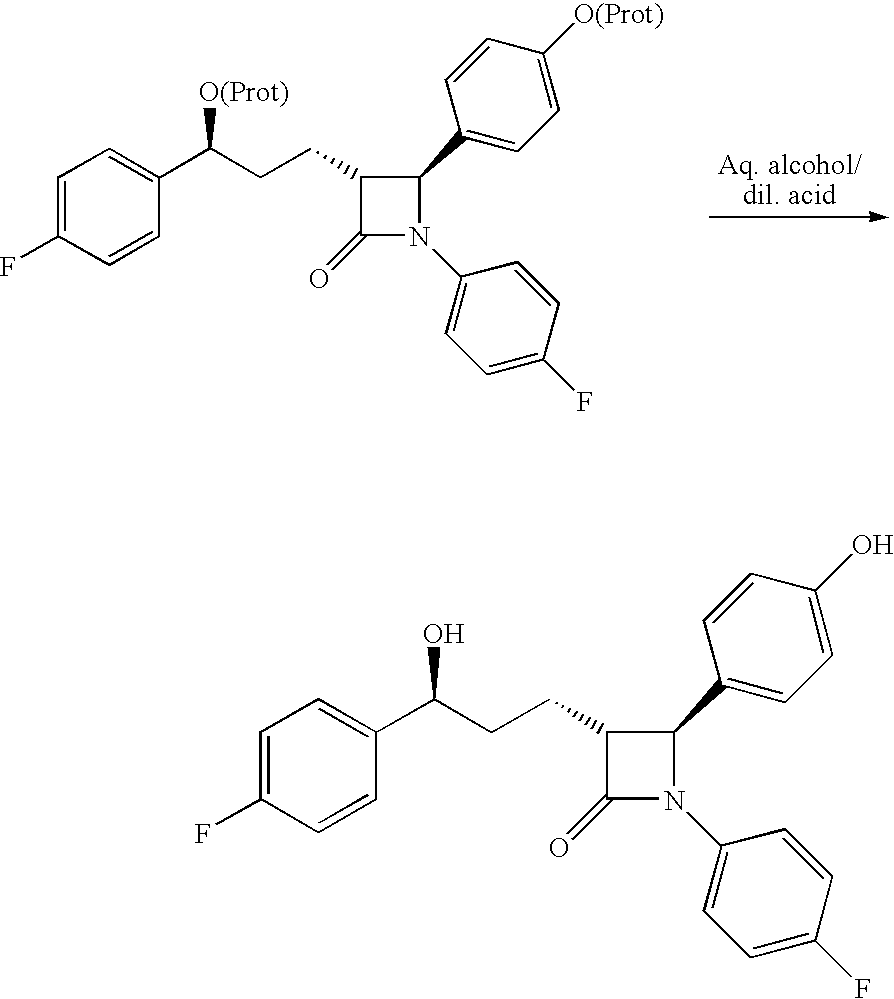
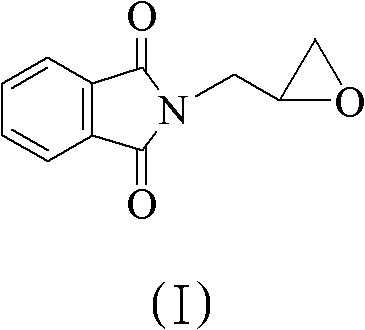
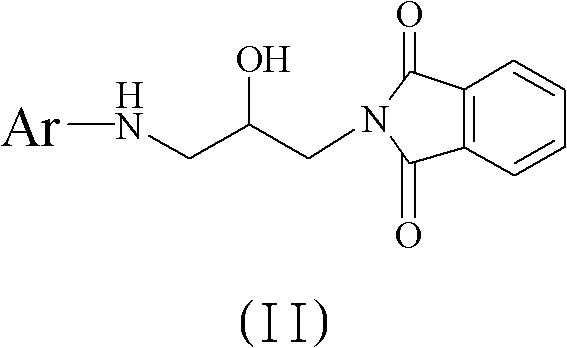
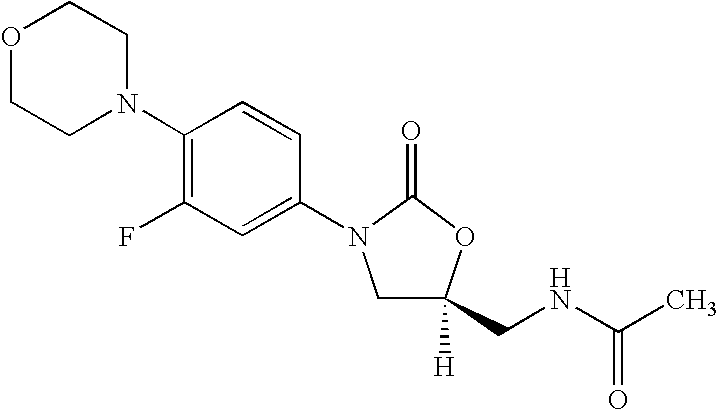
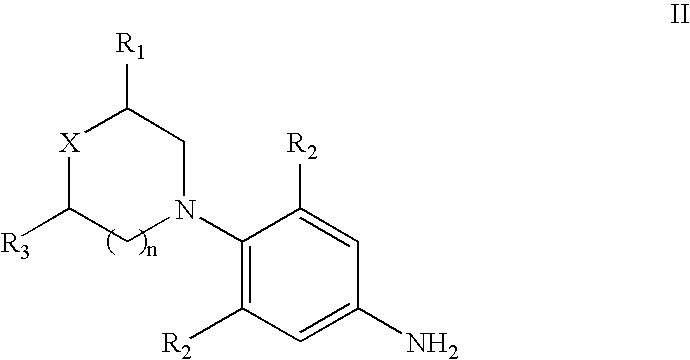
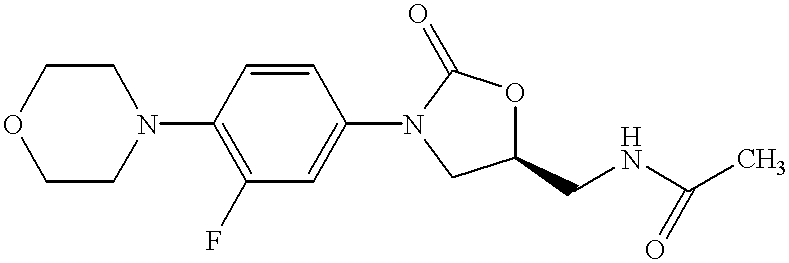
Novel process for the preparation of linezolid and related compounds
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
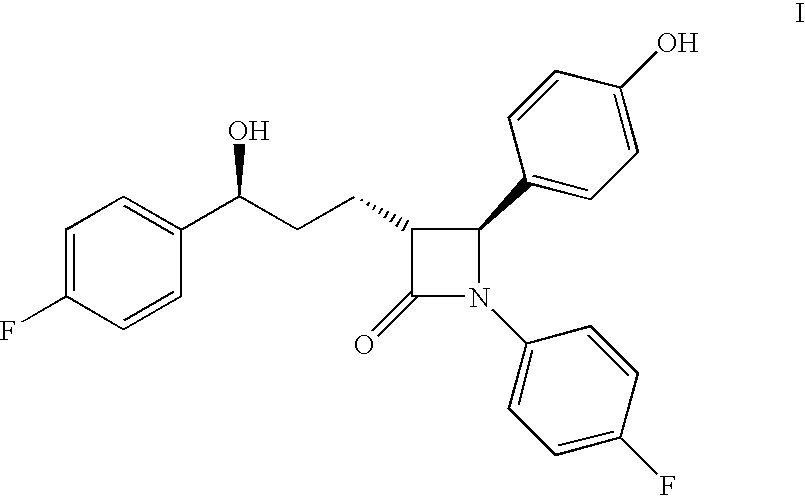
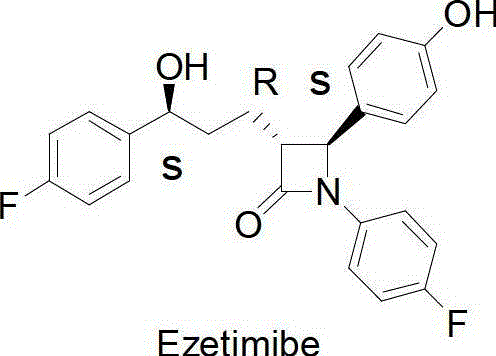
Process for ezetimibe intermediate
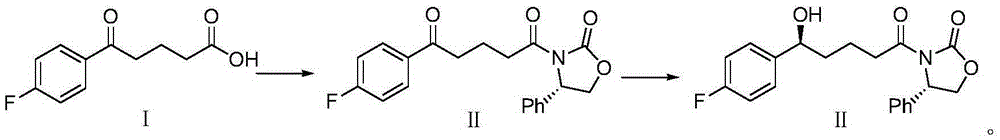
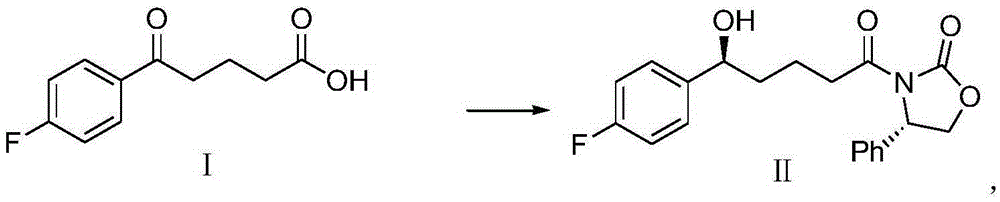
The invention provides a process for preparing intermediate of ezetimibe, which shows hypocholesterolemic activity. Thus 3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone is reduced with (−)-DIP chloride to obtain 3-[(5S)-5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-2-oxazolidinone.
Owner:HETERO DRUGS LTD
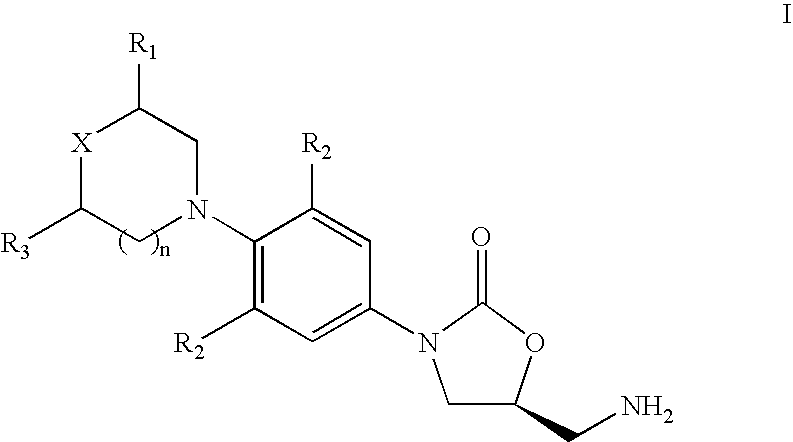
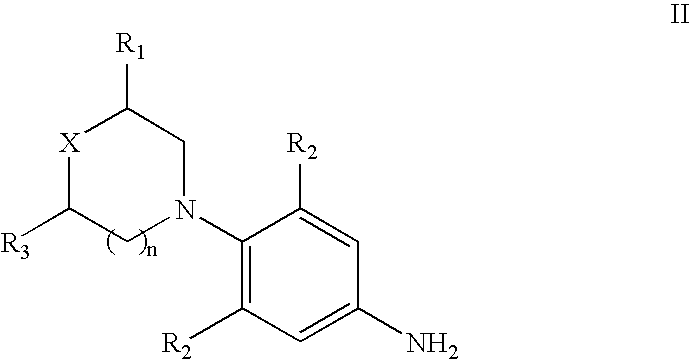
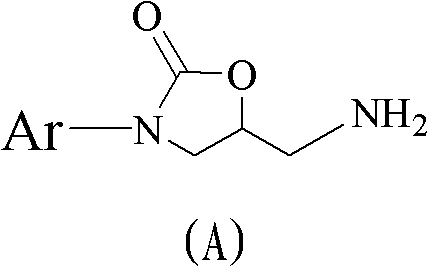
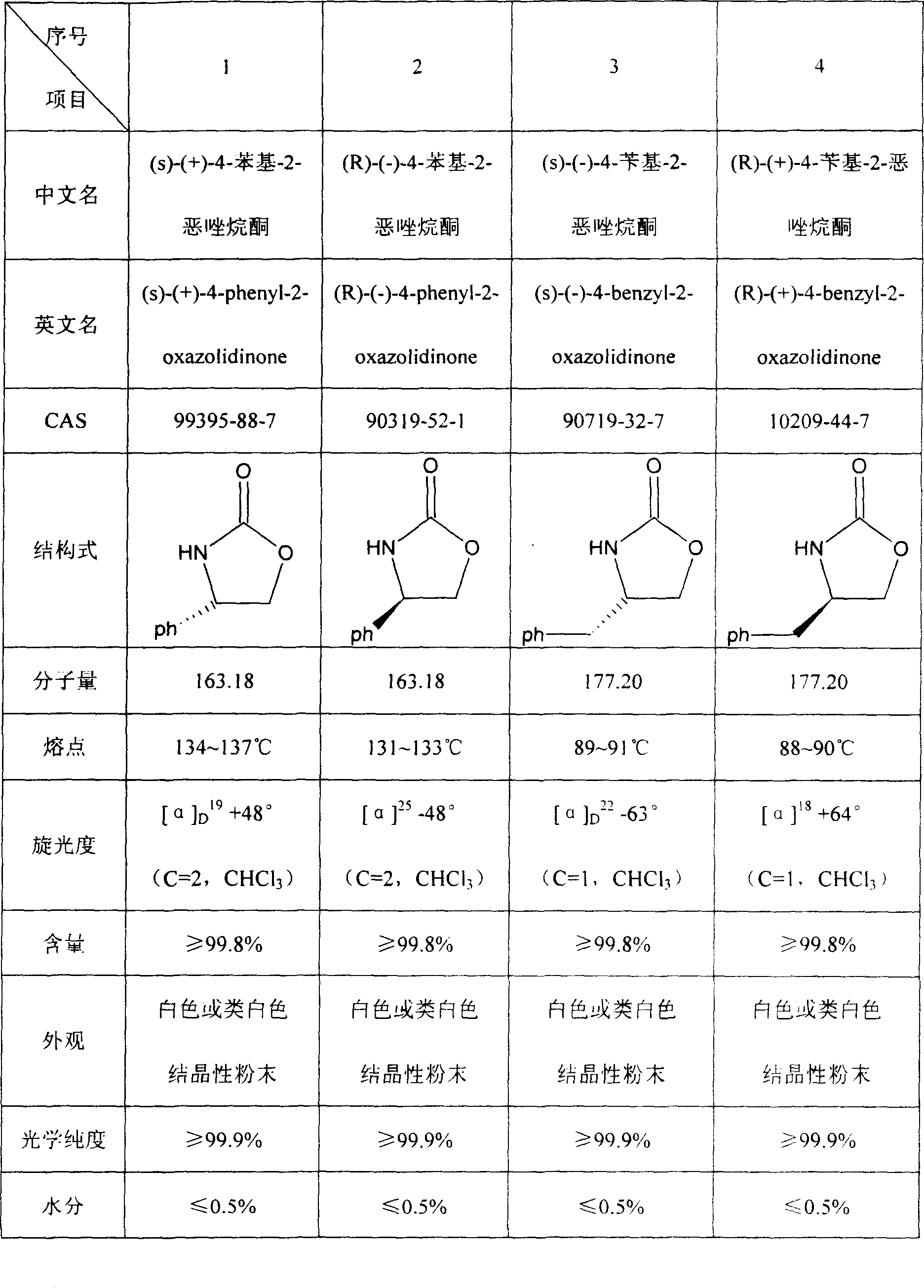
Method for preparing 5(S)-aminomethyl-3-aryl-2-oxazolidinone
The invention discloses a method for preparing 5(S)-aminomethyl-3-aryl-2-oxazolidinone. The method comprises the following steps of: reacting (S)-glycidyl phthalimide and aryl aniline to prepare an addition compound; and finally preparing the 5(S)-aminomethyl-3-aryl-2-oxazolidinone through cyclization reaction and aminolysis reaction. The method has the advantages of high total yield and low cost; all intermediates and a product synthesis method have the characteristics of convenience in recovery and purification; and the purity of a final product of the method is at least over 99.9 percent.
Owner:OMEGA MEDICAL TAIWAN
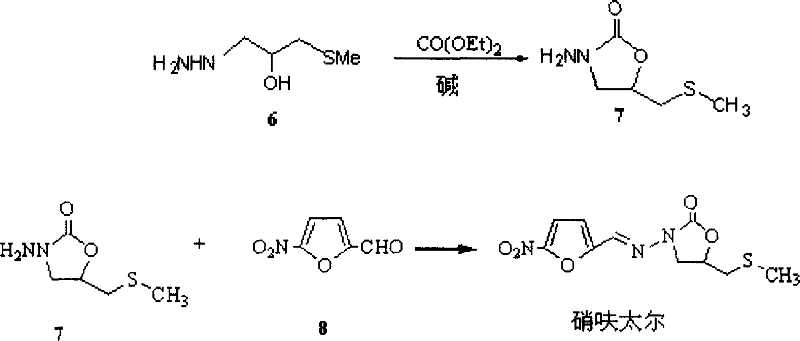
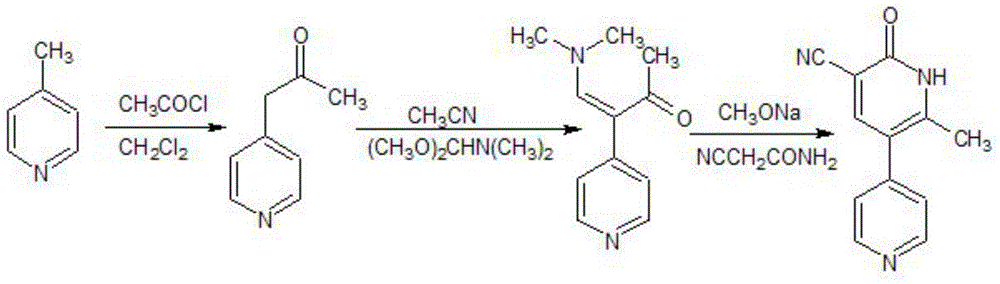
Production method of nifuratel
The invention relates to a medicine producing field, specifically, to an improved producing method of antibacterial medicine nifuratel. The method makes use of thiourea as initial material to produce nifuratel. The improvement is that it is no longer to use methyl mercaptan or methomyl in the known method while producing the intermediate 2-(methylthiomethyl)hydropropane. Furthermore, the invention is no longer to use metal natrium in the known method when the 3-methylthio-2-hydroxy-propylhydrazine and diethyl carbonate are heated to produce N-amido-5-methylthiomethyl-2-oxazolidone in the existance of alkali. All of the improvements largely improve the preparation of the nifuratel, especially for the production condition in a large scale, reduce the environment pollution and benefit to ensure the production safety.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
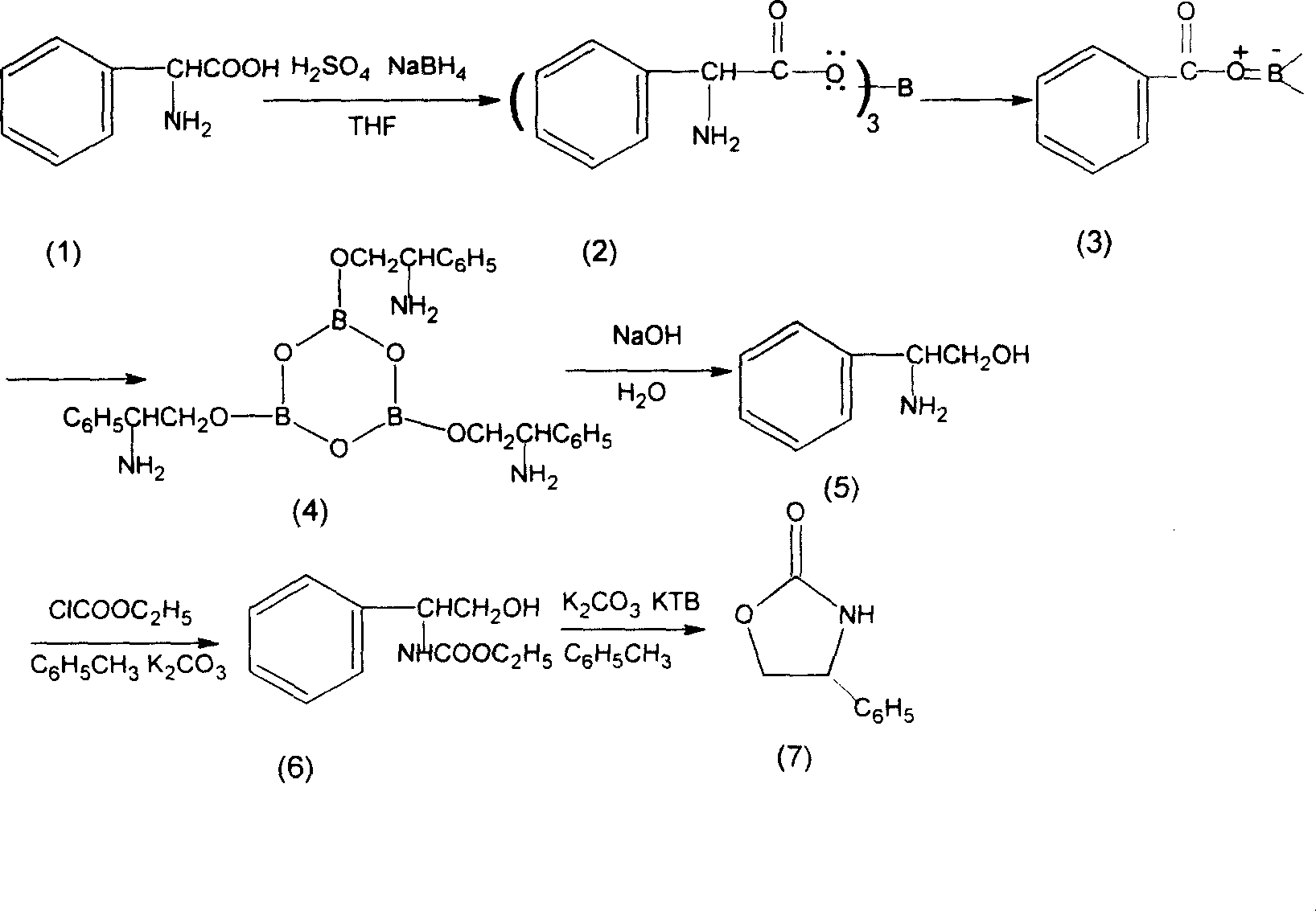
Prepn process of chiral 4-substituent-2-oxazolidone
InactiveCN1931847AMild reaction conditionsCommon reaction conditionsOrganic chemistryAlcoholMetallole
The present invention discloses the preparation process of one kind of chiral 4-substituent-2-oxazolidone. The preparation process includes the first reducing chiral amino acid into alcohol in the presence of Lewis acid and catalyst metal boron compound, the subsequent acylation with acyl chloride compound and no phosgene, and cyclization in buffering solution with strong alkali catalyst. The present invention has low cost, less reaction steps, less wastes produced, high product quality and high total yield, and is suitable for industrial production.
Owner:上海五洲药业股份有限公司 +1
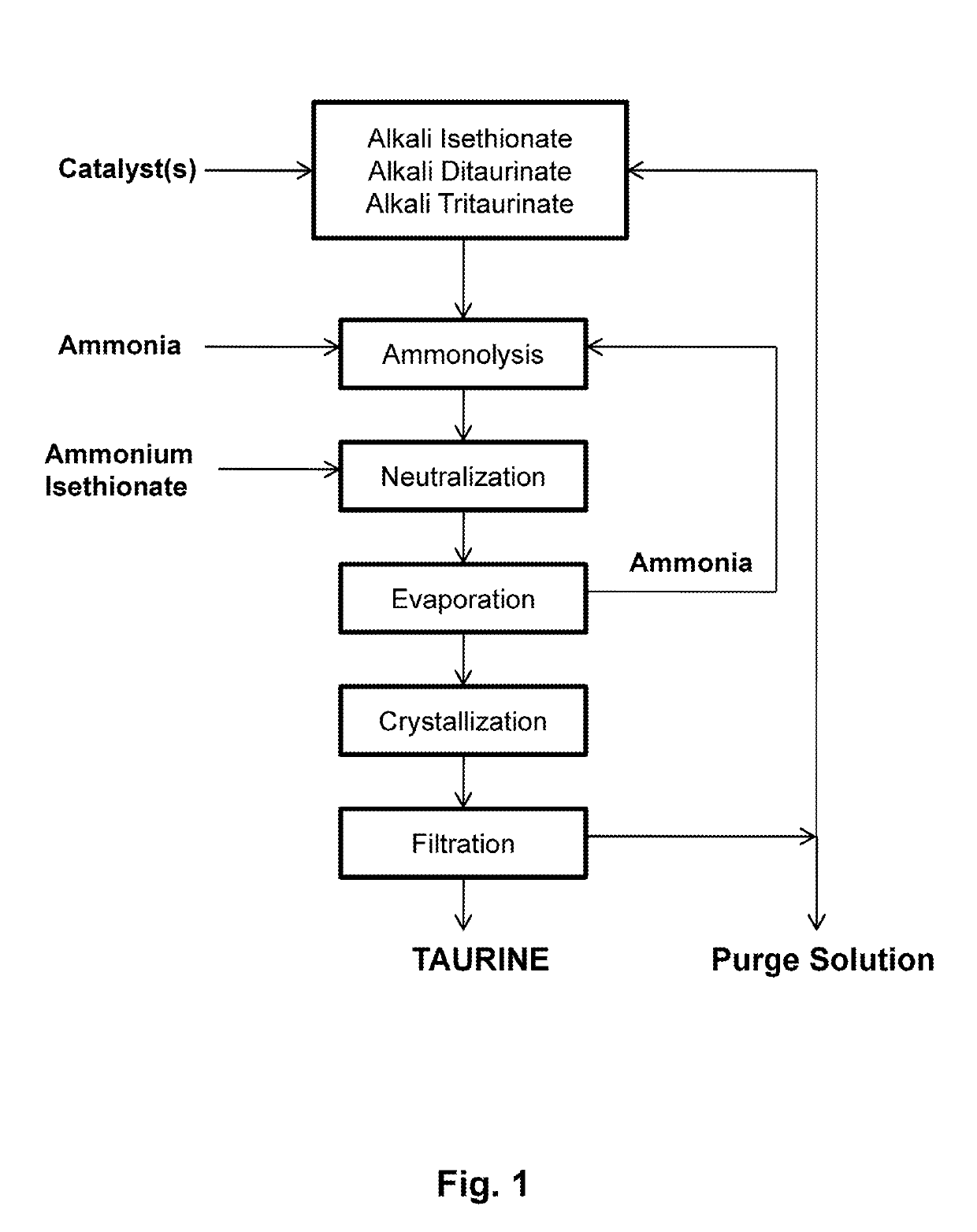
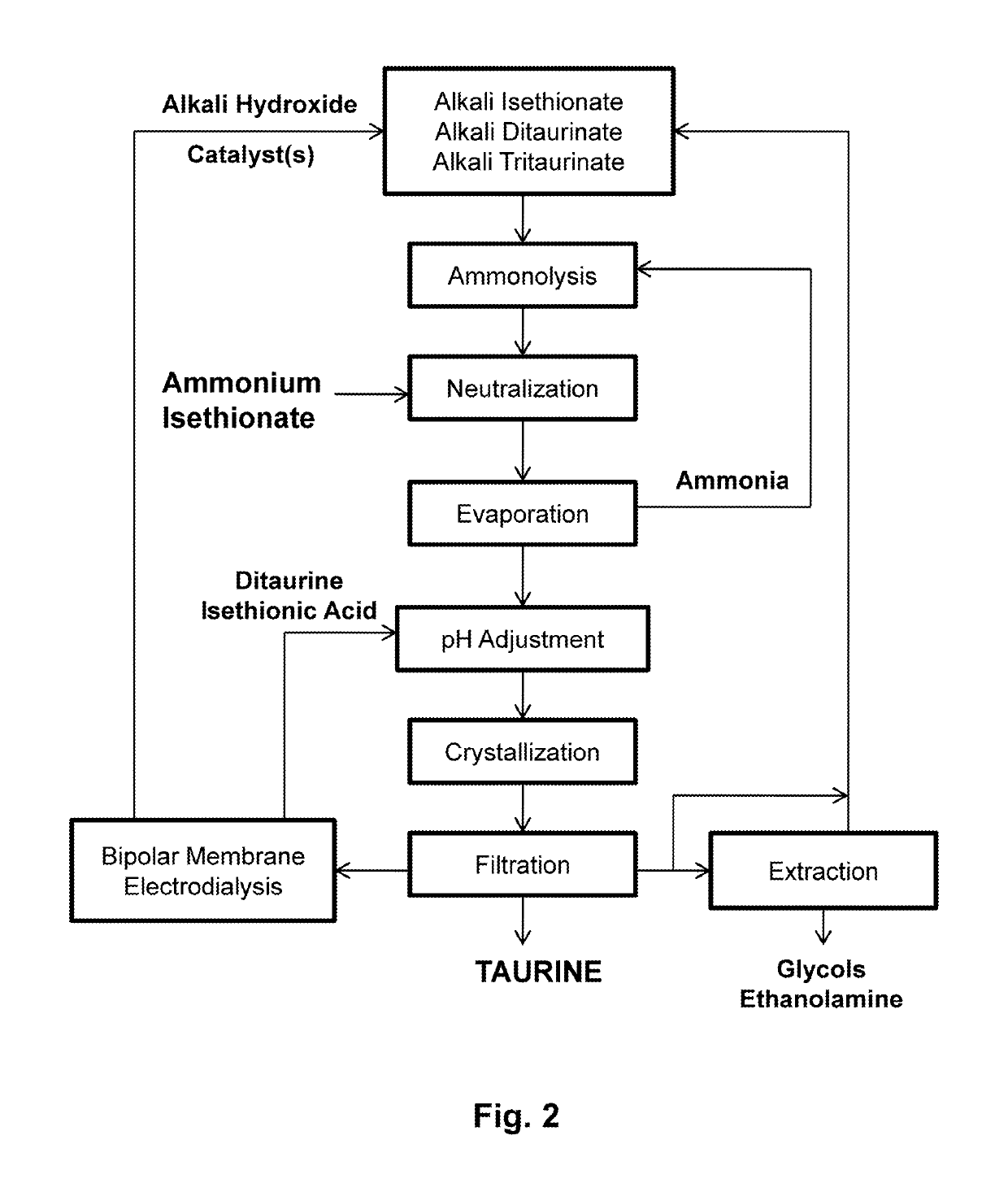
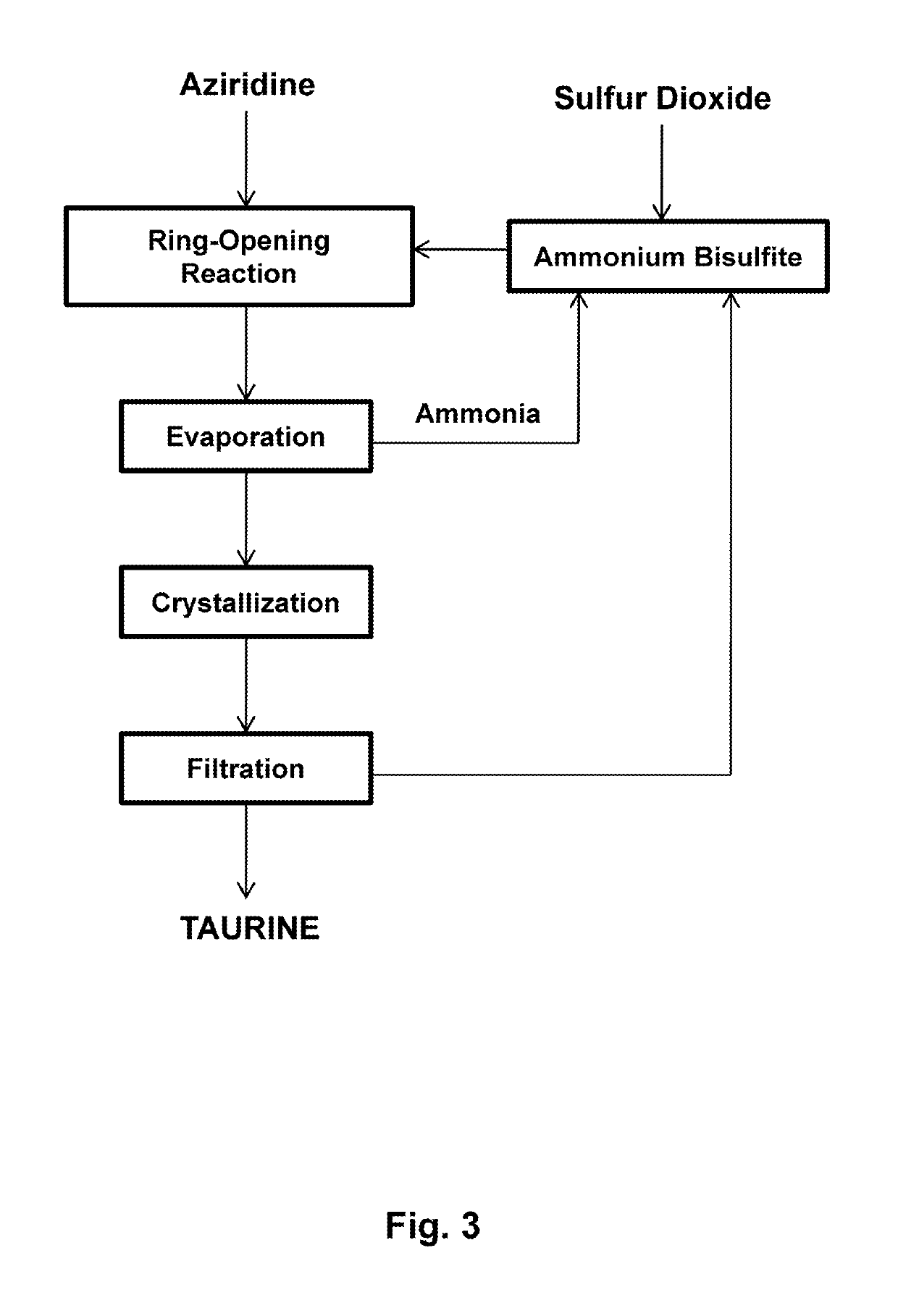
Process for producing taurine
ActiveUS20190135739A1Simple processOrganic compound preparationSulfonic acids salts preparationTaurineAqueous solution
There is disclosed a process for producing taurine by reacting 2-oxazolidinone with ammonium sulfite, or ammonium bisulfite, or a mixture of ammonium sulfite and ammonium bisulfite in an aqueous solution to form ammonium taurinate and ammonium bicarbonate. Taurine is obtained by decomposing ammonium taurinate to taurine and ammonia and recovered by solid-liquid separation.
Owner:VITAWORKS IP LLC
Process for the preparation of linezolid and related compounds
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Shaping keratin fibres using 2-oxazolidinone compounds
Described herein is a method for shaping keratin fibres including (i) providing a crosslinking composition, wherein the crosslinking composition includes a 2-oxazolidinone compound, a photocatalyst being a hydroxy-substituted aromatic compound, and a cosmetically acceptable carrier; (ii) applying the crosslinking composition to keratin fibres; and (iii) mechanically shaping the keratin fibres with an appliance at a temperature of from about 80° C. to about 180° C. while exposing the crosslinking composition to electromagnetic radiation having a wavelength of from about 300 nm to about 750 nm.
Owner:THE PROCTER & GAMBLE COMPANY
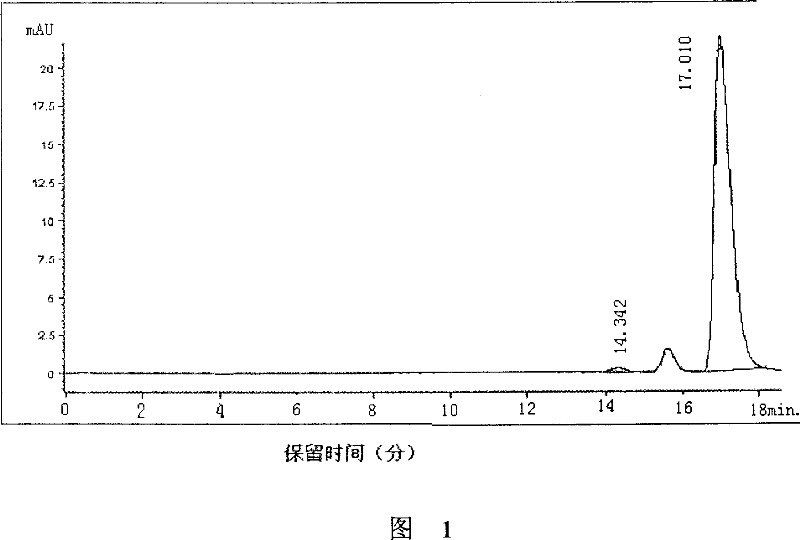
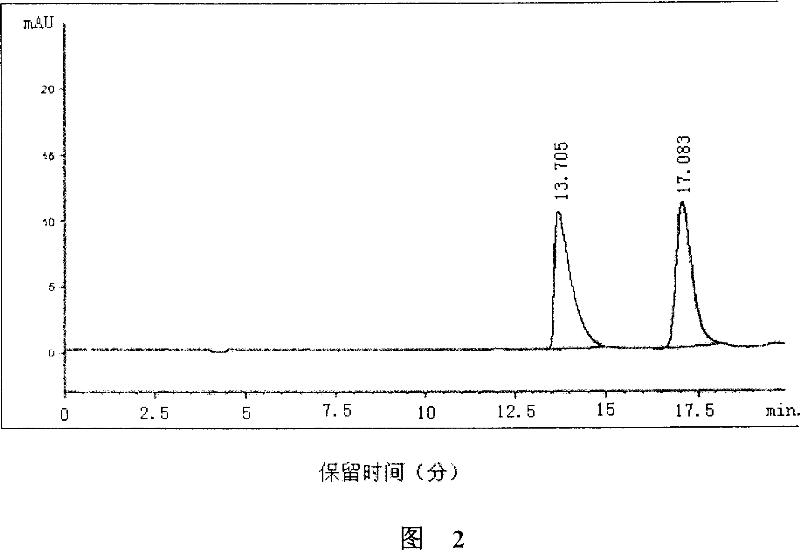
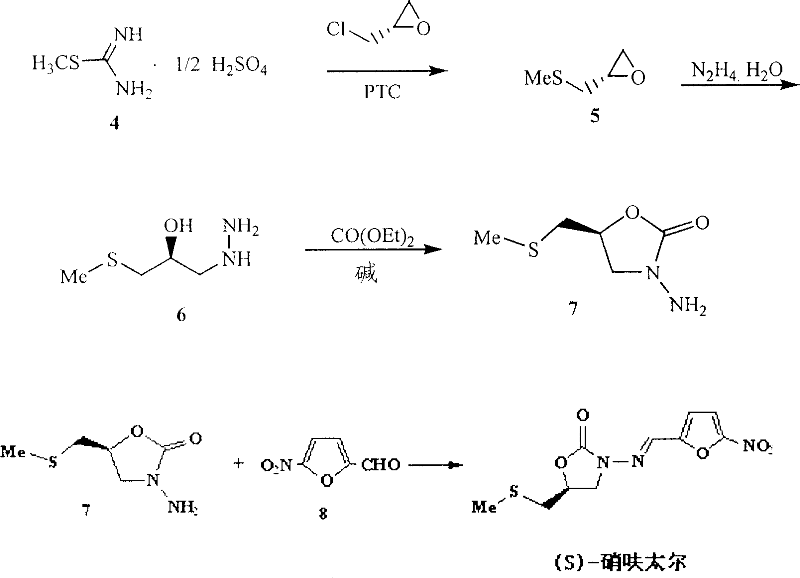
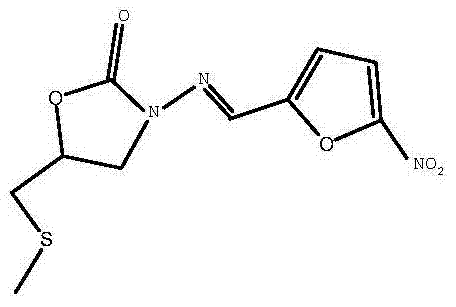
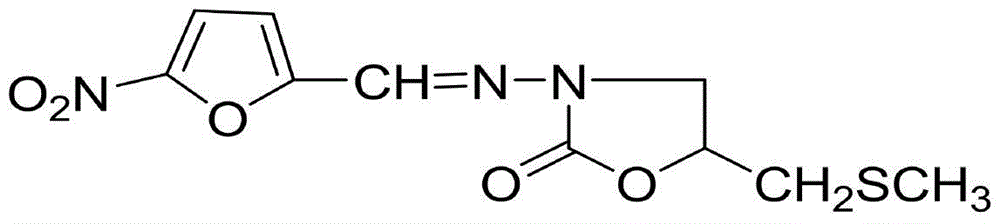
(S)-nifuratel, preparation method and application thereof
The invention discloses a nifuratel with a optical activity. Specifically, the invention provides a (S)-nifuratel, i.e. (S)-5-[(methylthio)methyl]-3-[(5-nitryl-2-furan)methylene]amido]-2-oxazolidone, the producing method and the combination including the optical activity compound. Compared to the racemic nifuratel, (S)-nifuratel has a better anti-inflammatory and anti-fungi activity. The invention also discloses the producing method for (S)-nifuratel which has the (S)-chloroepoxy propane as initial material, adopts the 3-dimension and selective synthesis method and produce a high optical purity (S)-nifuratel with a high yield.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
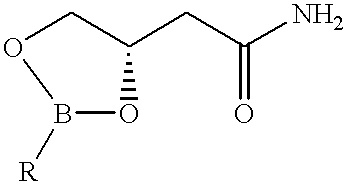
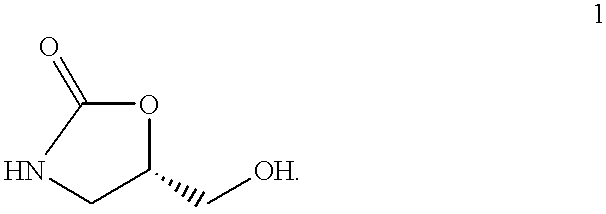
Process for the preparation of 5-hydroxymethyl 2-oxazolidinone and novel intermediate
A process for preparing 5-hydroxymethyl-2-oxazolidinone (1), preferably optically active, in one step from 3,4-boronic acid ester protected 3,4-dihydroxybutyramides (2) is described. The oxazolidinone is important in the pharmaceutical industry especially in the areas of antimicrobials and behavioral disorders.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
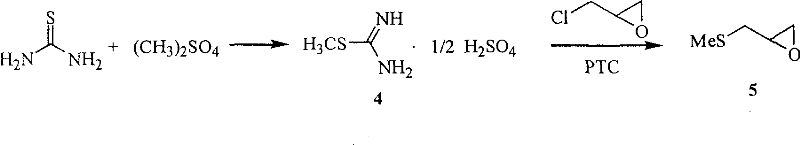
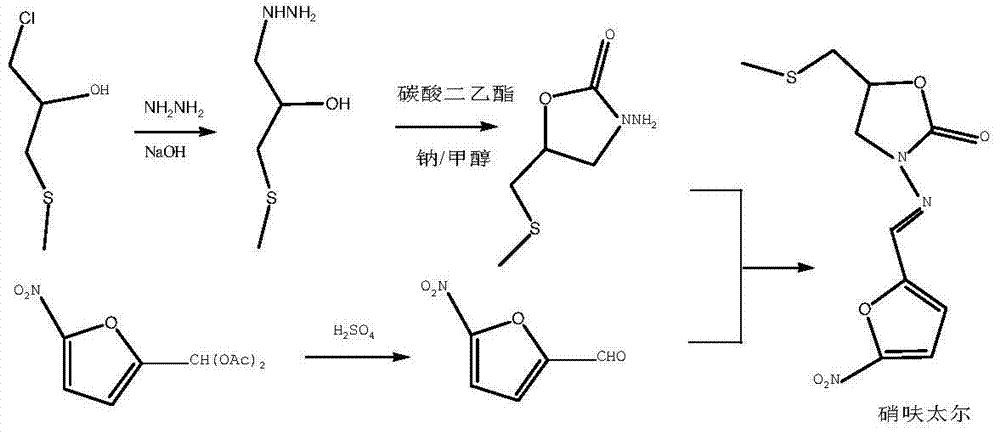
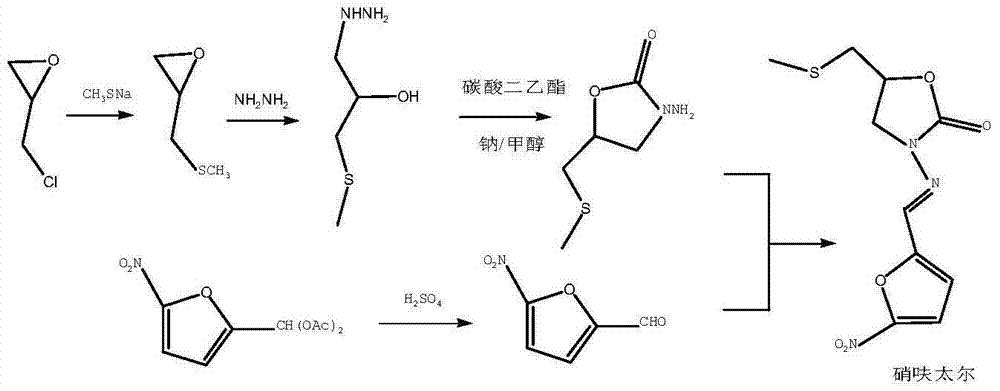
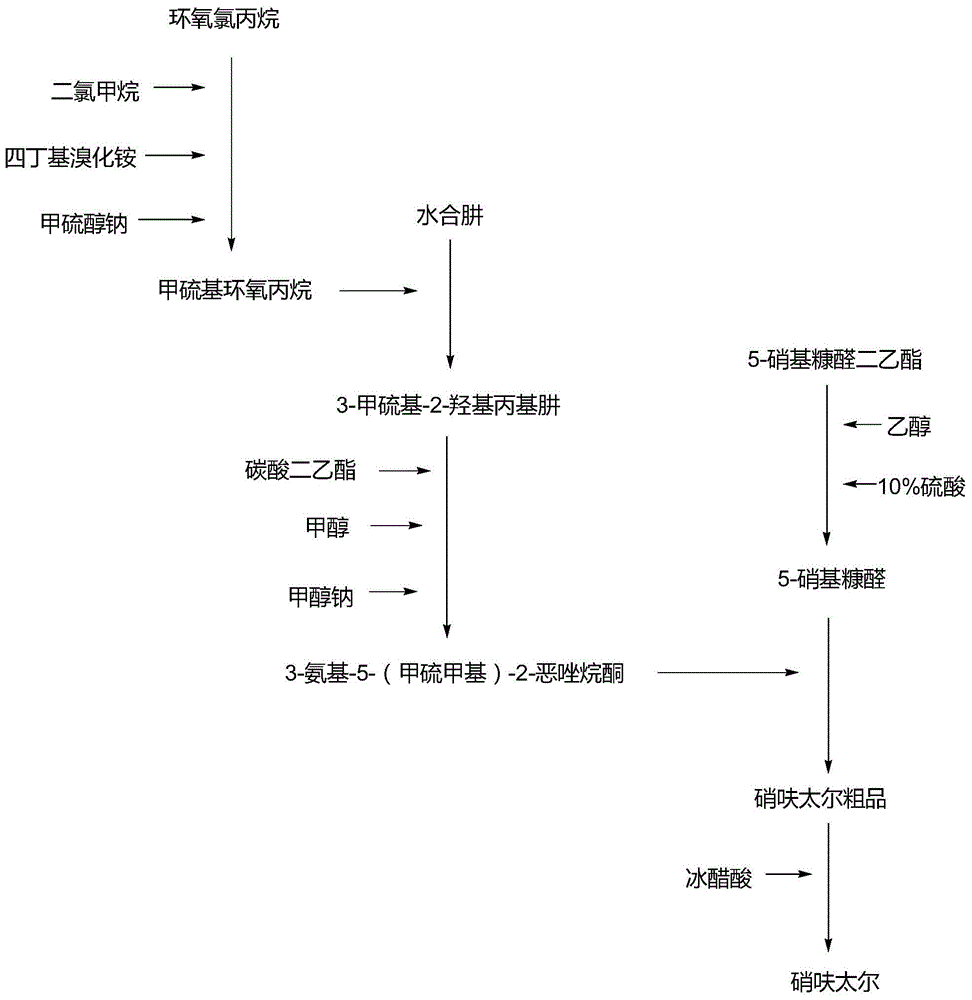
Preparation method for nifuratel
The invention relates to a method for preparing nifuratel. The method comprises the steps of (1) synthesizing 2-(methylmercapto-methyl)-oxacyclopropane through epoxy chloropropane and sodium methyl mercaptide; (2) generating reaction between hydrazine hydrate and the 2-(methylmercapto-methyl)-oxacyclopropane to synthesize 3-methylmercapto-2-hydroxy-propyl hydrazine; (3) generating reaction between diethyl carbonate and 3-methylmercapto-2-hydroxy-propyl hydrazine to prepare N-amino-5-methylmercapto-methyl-2-oxazolidinone; (4) hydrolyzing 5-nitrofuran formaldehyde diacetate ester under an acidic condition to prepare 5-nitro-2-furaldehyde; (5) generating reaction between the 5-nitro-2-furaldehyde and the N-amino-5-methylmercapto-methyl-2-oxazolidinone obtained in the step (3) to obtain the nifuratel, wherein in the step (1), 15-crown ether-5 is used as a catalyst, so that the conversion rate is high; no organic solvent is used during posttreatment of a product, and the posttreatment is simple.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Detection kit and method of furazolidone metabolin
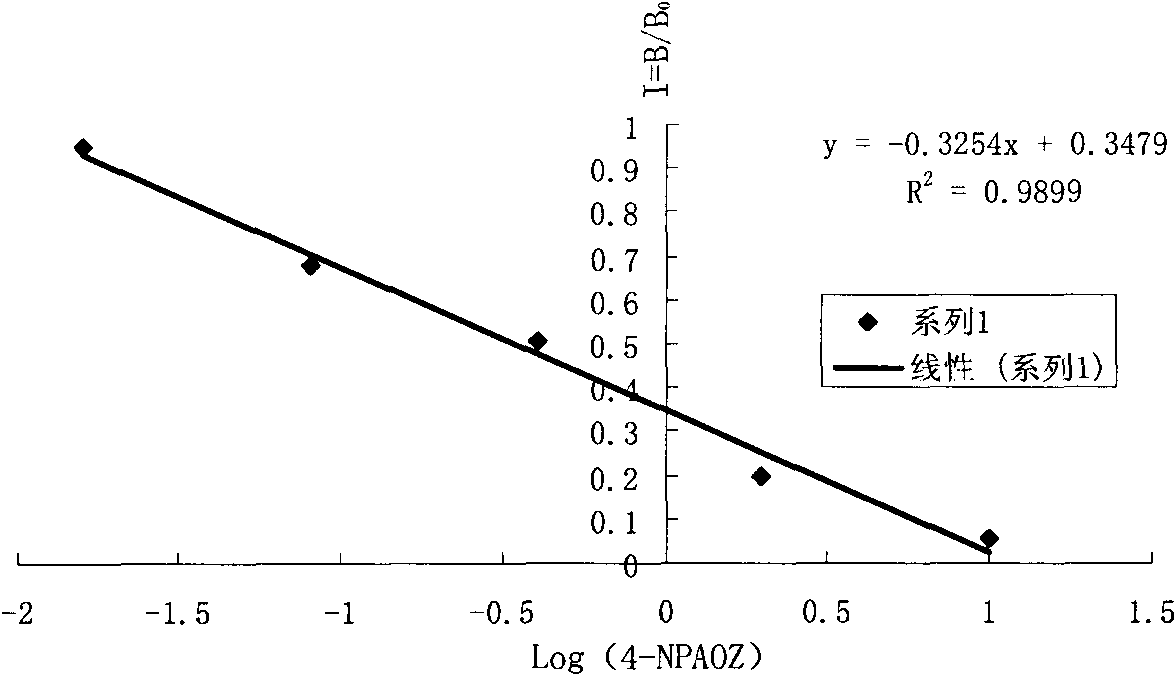
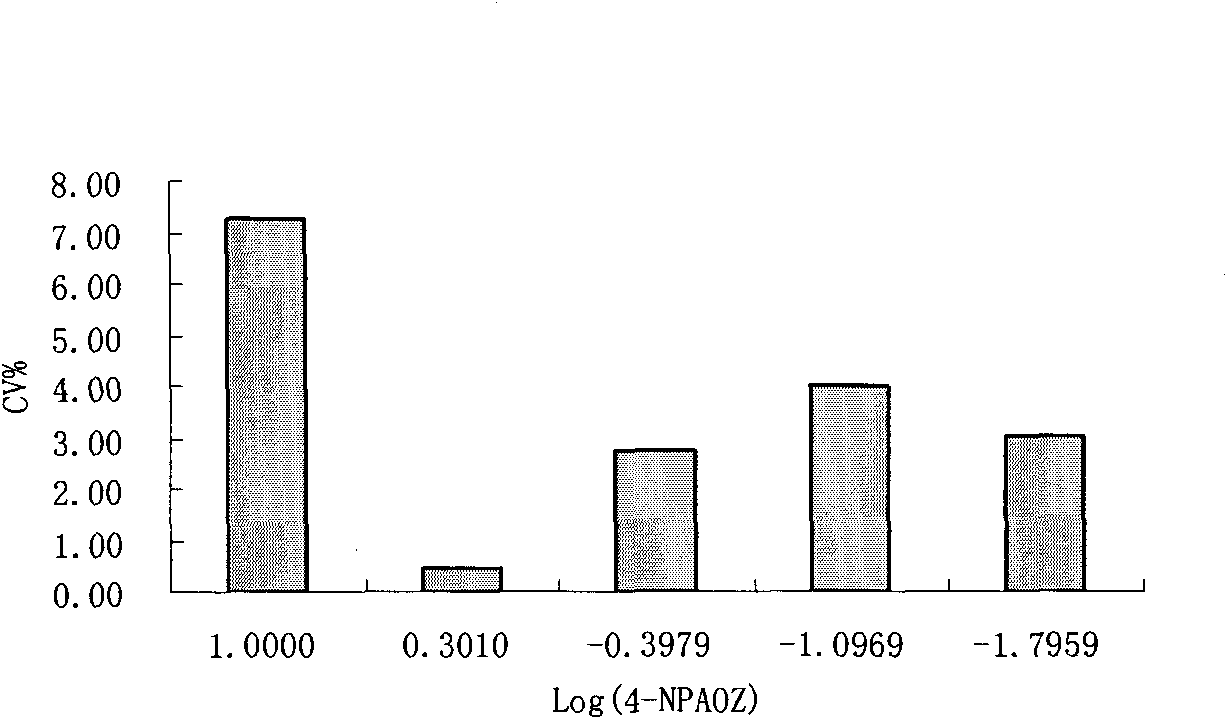
InactiveCN101806796AImprove featuresIncreased sensitivityChemiluminescene/bioluminescenceBiological testing3-amino-2-oxazolidoneP-Nitrobenzene
The invention discloses a chemiluminescence detection kit of furazolidone metabolin, comprising a detection plate and a reagent, wherein the reagent comprises an antibody and a detection target standard solution, wherein the detection target is a p-nitrobenzene derivative 4-NPAOZ of furazolidone metabolin 3-amino-2-oxazolidone. The detection kit of the furazolidone metabolin not only can realize the fast and mass detection, but also has very high specificity and sensitivity, and achieves the detection sensitivity of 0.005 ppb.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI +2
Hydantoin-enhanced halogen efficacy in pulp and paper applications
InactiveUSRE39021E1Improve bactericidal efficacyReduce the amount requiredBiocideNatural cellulose pulp/paperHydantoinSuccinimide
Free halogen sources (e.g., sodium hypochlorite and chlorine) added as slimicides in high organic component process streams such as pulp and paper processing are rendered more efficacious by the addition of selected N-hydrogen compounds (namely, 5,5-dimethylhydantoin, 5-ethyl-5-methylhydantoin, cyanuric acid, succinimide, urea, 4,4-dimethyl-2-oxazolidinone, and glycouril) to the process stream. The latter compounds may be added to the process stream before or after the slimicide is added or combined with the slimicide and added directly thereto. The direct use of halogenated hydantoins has also been found to provide improved efficacy relative to free halogen sources. In addition, absorbable organic halogen by-products are reduced.
Owner:LONZA INC
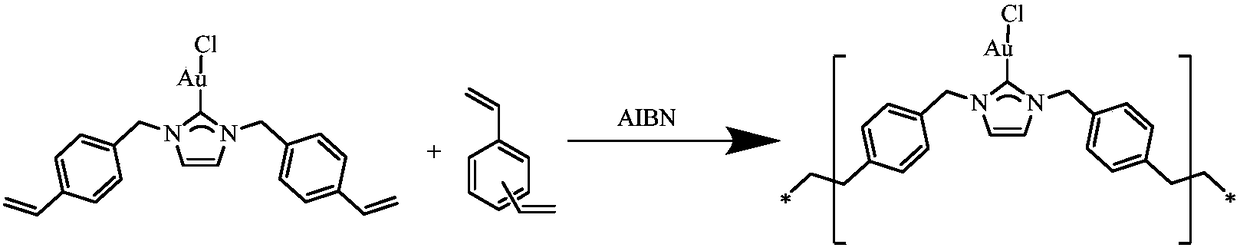
Preparation and application of N-heterocyclic carbene gold porous organic polymer
InactiveCN108822243ALow toxicity areaLow Toxic Pore SizeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCarbeneDivinyl benzene
Owner:EAST CHINA NORMAL UNIV
Process for preparing carbamate, urea and their derivatives as well as 2-oxzolidone
InactiveCN1900055AHigh selectivityEasy to manufactureUrea derivatives preparationCarbamic acid derivatives preparationCarbamatePhosphate
The present invention discloses preparation of nitrogen containing carbonyl compound, and is especially the process of preparing carbamate, urea and their derivatives as well as 2-oxazolidone through oxidizing and carbonylating organic amine compound in the presence of one kind of multifunctional catalyst. By using salt of VIII metal and Cu as main metal catalyst and the ionic solution of halogenated pyridine salt, quaternary ammonium salt or quaternary phosphate salt as co-catalyst and through oxidizing and carbonylating organic amine compound, carbamate, urea and their derivatives as well as 2-oxazolidone may be prepared at oxygen pressure of 0.2-1.0 MPa, CO pressure of 1.0-4.0 MPa, reaction temperature of 80-220 deg.c, and in reaction time of 0.5-4 hr. Compared with the technological process adopting traditional catalyst, the present invention has the advantages of simple system, easy preparation, mild reaction condition and high selectivity.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
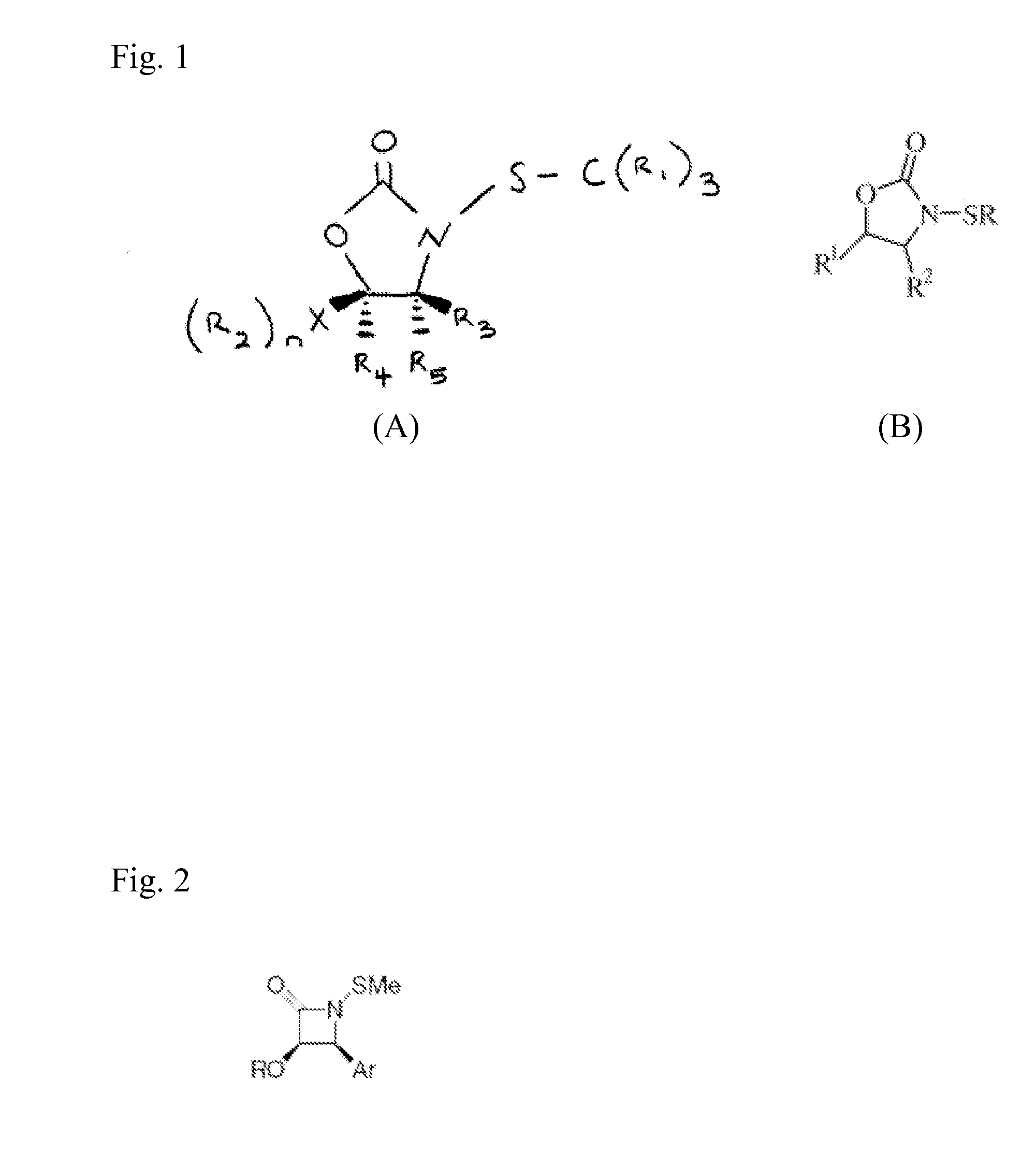
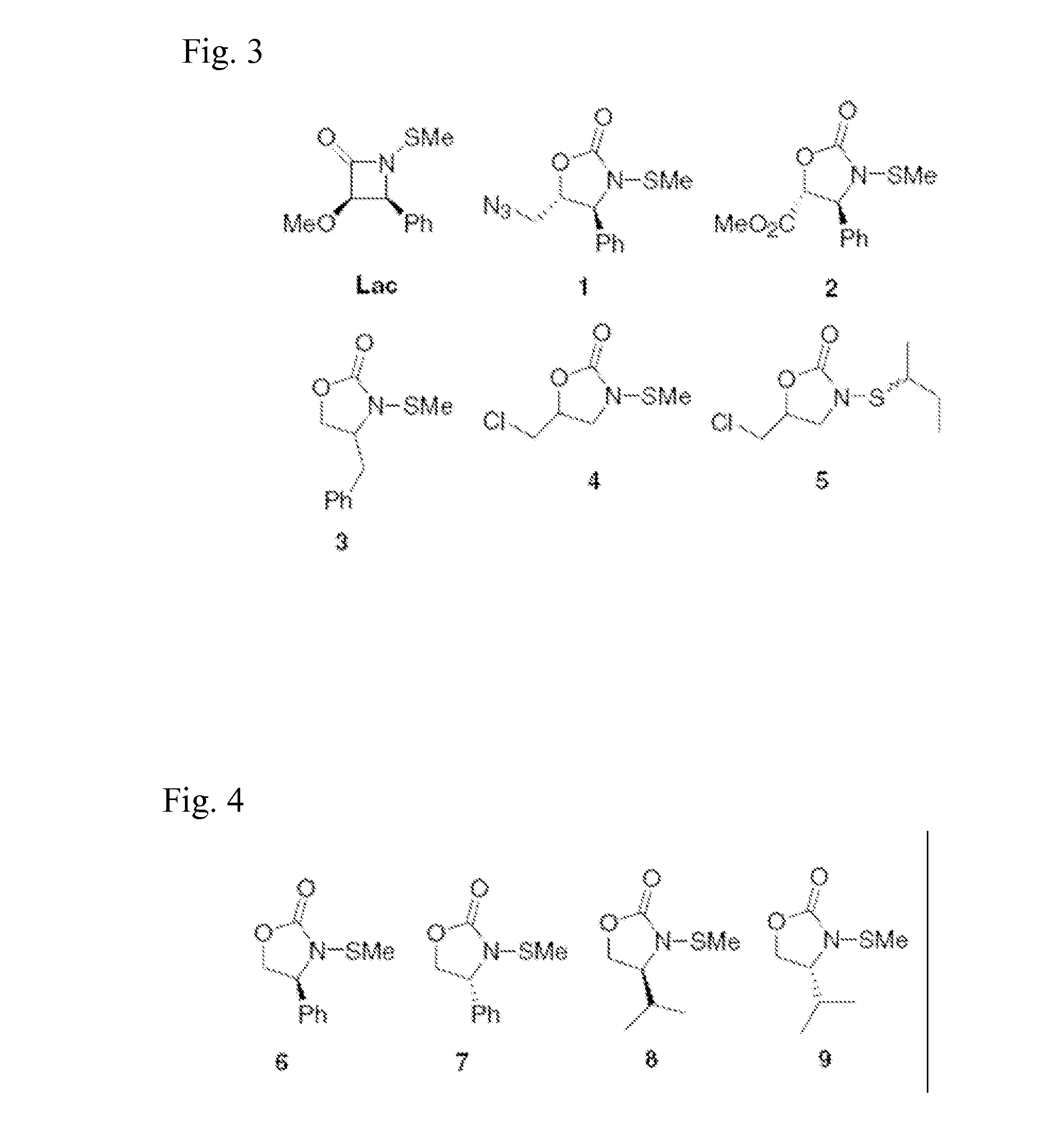
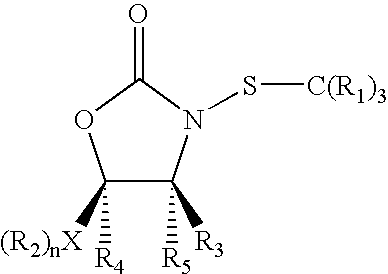
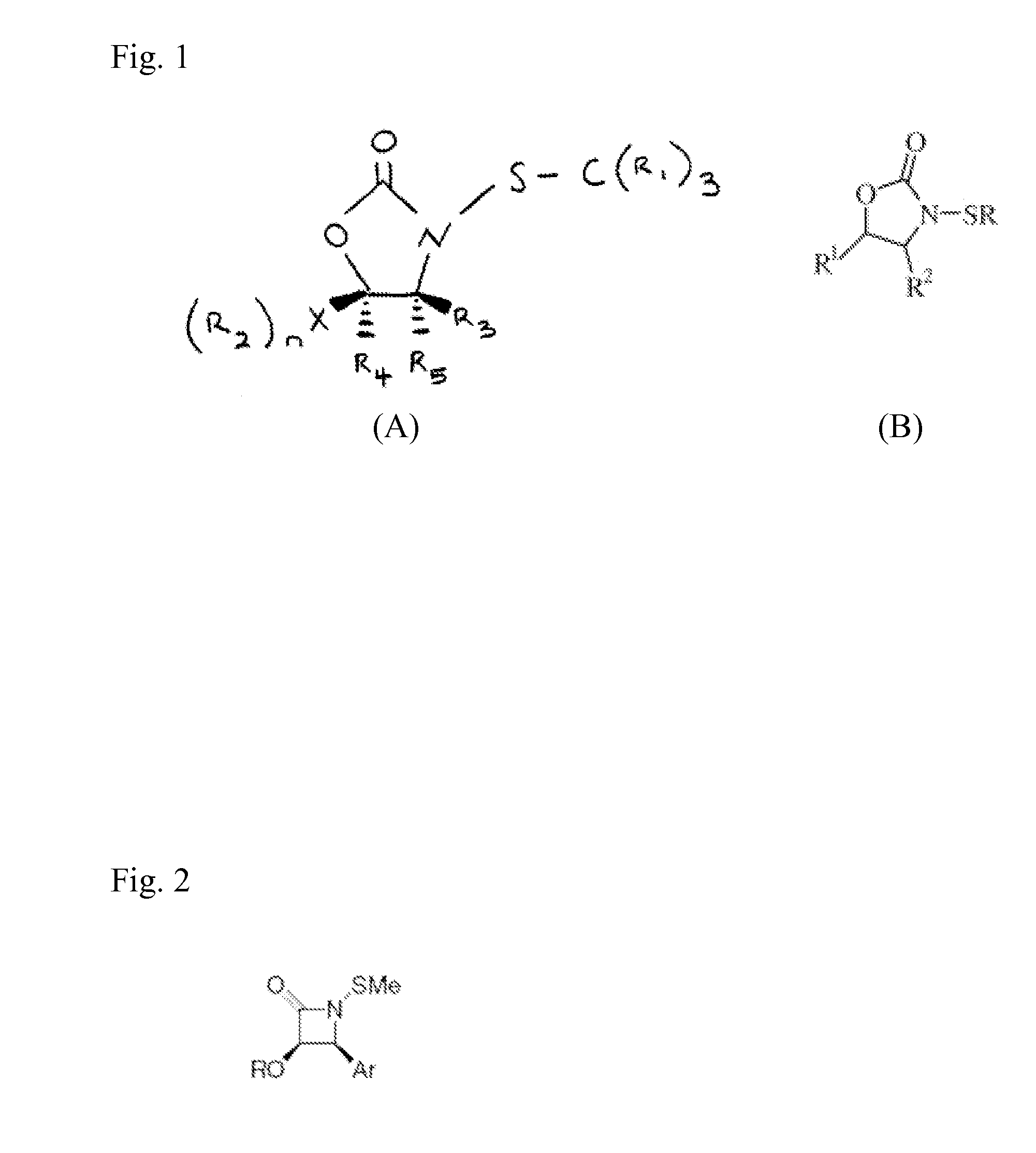
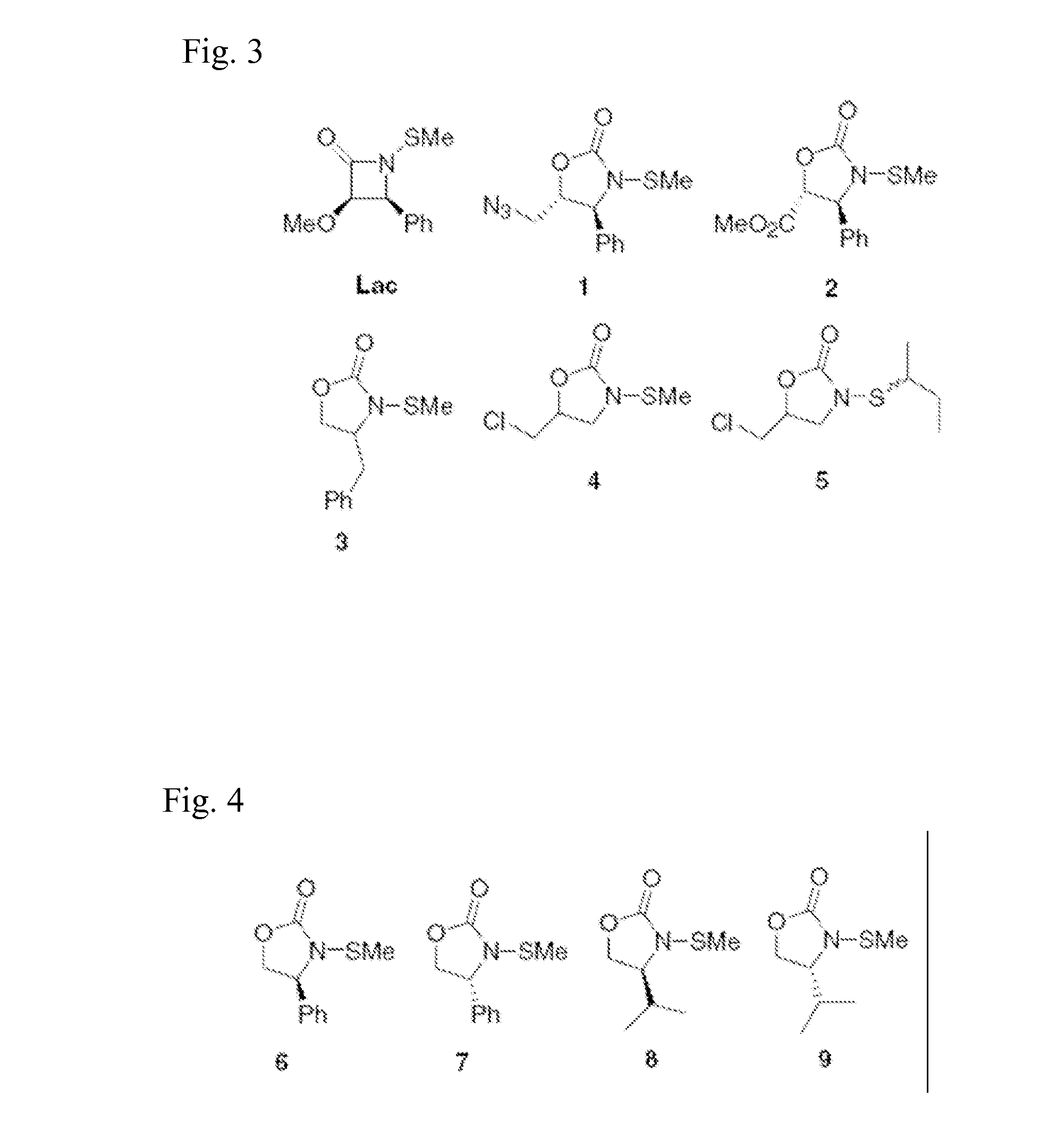
N-Thiolated 2 Oxazolidone Antibiotics
This invention describes the discovery and synthesis of N-thiolated 2-oxazolidinones as a new class of anti bacterial agents. These compounds can be synthesized from 2-oxazolidinones by Ndeprotection and N-sulfenylation. These new substances were found to exhibit potent anti-bacterial activity, including bacteriostatic properties against Staphylococcus spp., including methicillin resistant Staphylcoccus aureus (MRSA), and Bacillus spp., including Bacillus anthracis.
Owner:UNIV OF SOUTH FLORIDA
Safe environment-friendly ink detergent and preparation method thereof
InactiveCN103525162AImprove craftsmanshipReduce pollutionChemical paints/ink removersPolyoxyethylene castor oilGluconic acid
The invention discloses a safe environment-friendly ink detergent. The invention is characterized in that the detergent is prepared from the following raw materials in parts by weight: 4-5 parts of polyoxyethylene sorbitan fatty acid ester, 4-5 parts of polyoxyethylene castor oil, 6-8 parts of acetone, 4-6 parts of ethyl acetate, 2-3 parts of sodium gluconate, 1-2 parts of triethanolamine, 3-4 parts of isopropyl myristate, 1-2 parts of polydimethylsiloxane, 1-2 parts of 2-oxazolidone, 4-5 parts of magnesium sulfate, 4-5 parts of coconut oil fatty acid, 2-3 parts of tall oil fatty acid, 4-5 parts of assistant and 100-110 parts of water. The assistants are added to improve the technical properties of the detergent and reduce the toxic or side effect and environmental pollution; and the detergent has the advantages of excellent dissolution and dilution capability for inks, no toxicity, no volatilization, no harm to the human body, no corrosivity and low manufacturing cost, and is convenient to use.
Owner:HEFEI ALL ROUND POLYMER MATERIAL FACTORY
Method for synthesising ezetimibe intermediate
InactiveCN102978253ASimple post-processingEasy to routeOrganic chemistryFermentationOrganic solventPhosphate
The invention provides a method for synthesising ezetimibe intermediate, comprising the following steps of: dissolving (4S)-3-[5-(4-fluorophenyl)-1,5-di-oxo-pentyl]-4-phenyl-2-oxazolidone and chiral carbonyl reductase in a two-phase mixed solution which is composed of an organic solvent and phosphate buffer; and performing synthesis reaction for 10-30 hours in an nitrogen protection environment, and then obtaining (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxo-pentyl]-4-phenyl-2-oxazolidone, wherein the temperature of the synthesis reaction is 20-50 DEG C, and the pH of the two-phase mixed solution is 5.5-8.5. Via the method, the technical problems of complex synthesis process and high production cost of (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxo-pentyl]-4-phenyl-2-oxazolidone in the prior art are solved.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Technology for producing nifuratel
The invention relates to a production process of nifuratel, which comprises the following steps: dissolving epichlorohydrin in dichloromethane, adding tetrabutylammonium bromide, adding sodium methyl mercaptide aqueous solution dropwise, extracting, depressurizing Distillation to obtain methylthio propylene oxide; add methylthio propylene oxide dropwise to hydrazine hydrate under heating conditions, and obtain 3-methylthio-2-hydroxypropyl hydrazine through vacuum distillation; 3-methylthio Base-2-hydroxypropylhydrazine is mixed with diethyl carbonate, sodium methoxide absolute ethanol solution is added, after a ring closure reaction occurs under reflux conditions, vacuum distillation is added, and absolute ethanol is added to obtain (3-amino-5-( Methylthiomethyl)-2-oxazolidinone) absolute ethanol solution, then dropwise added to 5-nitrofurfural, stirred and reacted, filtered, washed with methanol, and dried to obtain the crude product of nifuratel; refined and dried to obtain Nifuratel. The invention has easy-to-obtain raw materials, low cost, good yield, mild reaction conditions, easy mastery and convenient industrial production.
Owner:ANHUI YOUCARE KAIYUE PHARMA
N-thiolated 2 oxazolidone antibiotics
This invention describes the discovery and synthesis of N-thiolated 2-oxazolidinones as a new class of anti bacterial agents. These compounds can be synthesized from 2-oxazolidinones by Ndeprotection and N-sulfenylation. These new substances were found to exhibit potent anti-bacterial activity, including bacteriostatic properties against Staphylococcus spp., including methicillin resistant Staphylcoccus aureus (MRSA), and Bacillus spp., including Bacillus anthracis.
Owner:UNIV OF SOUTH FLORIDA
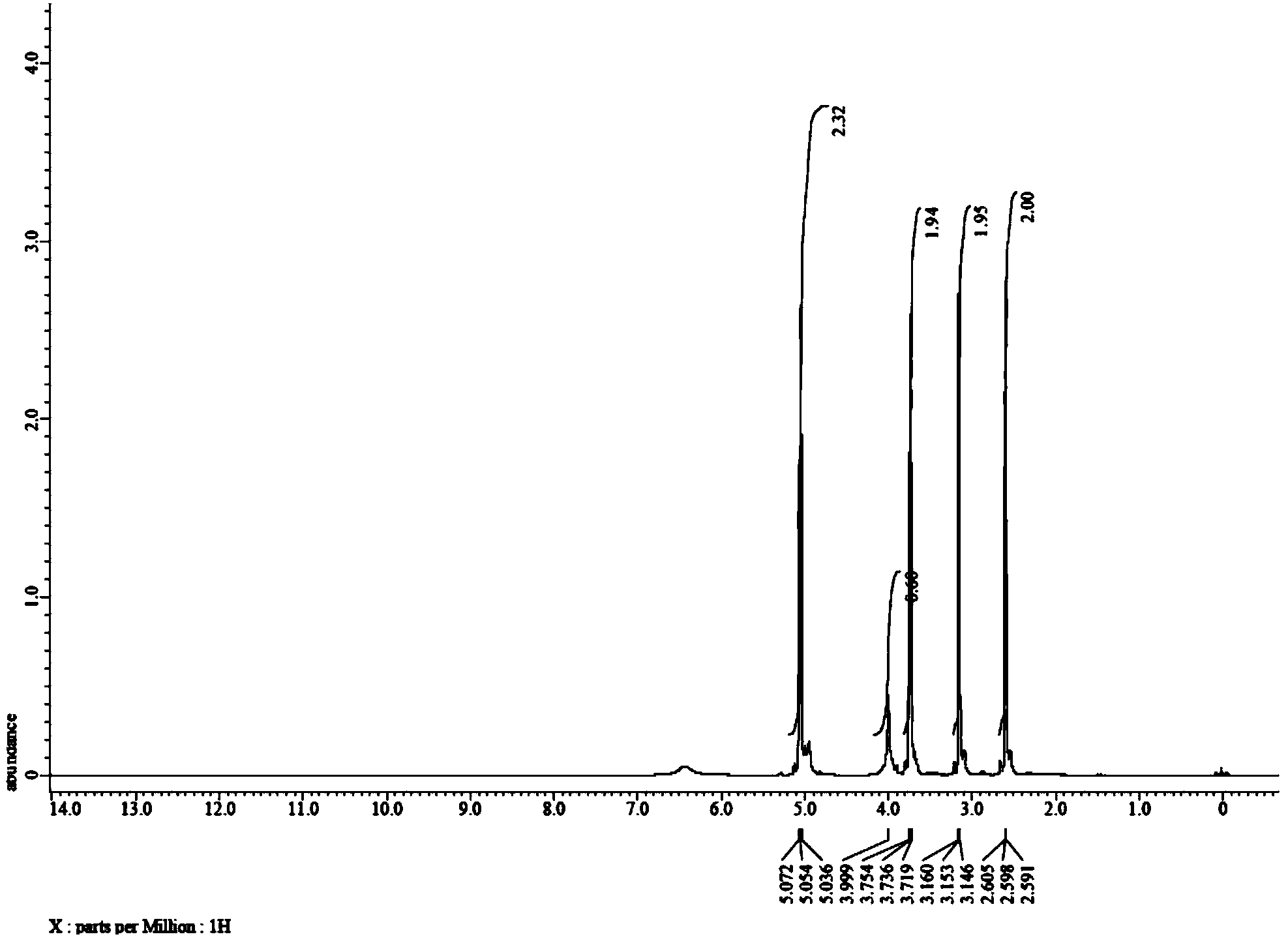
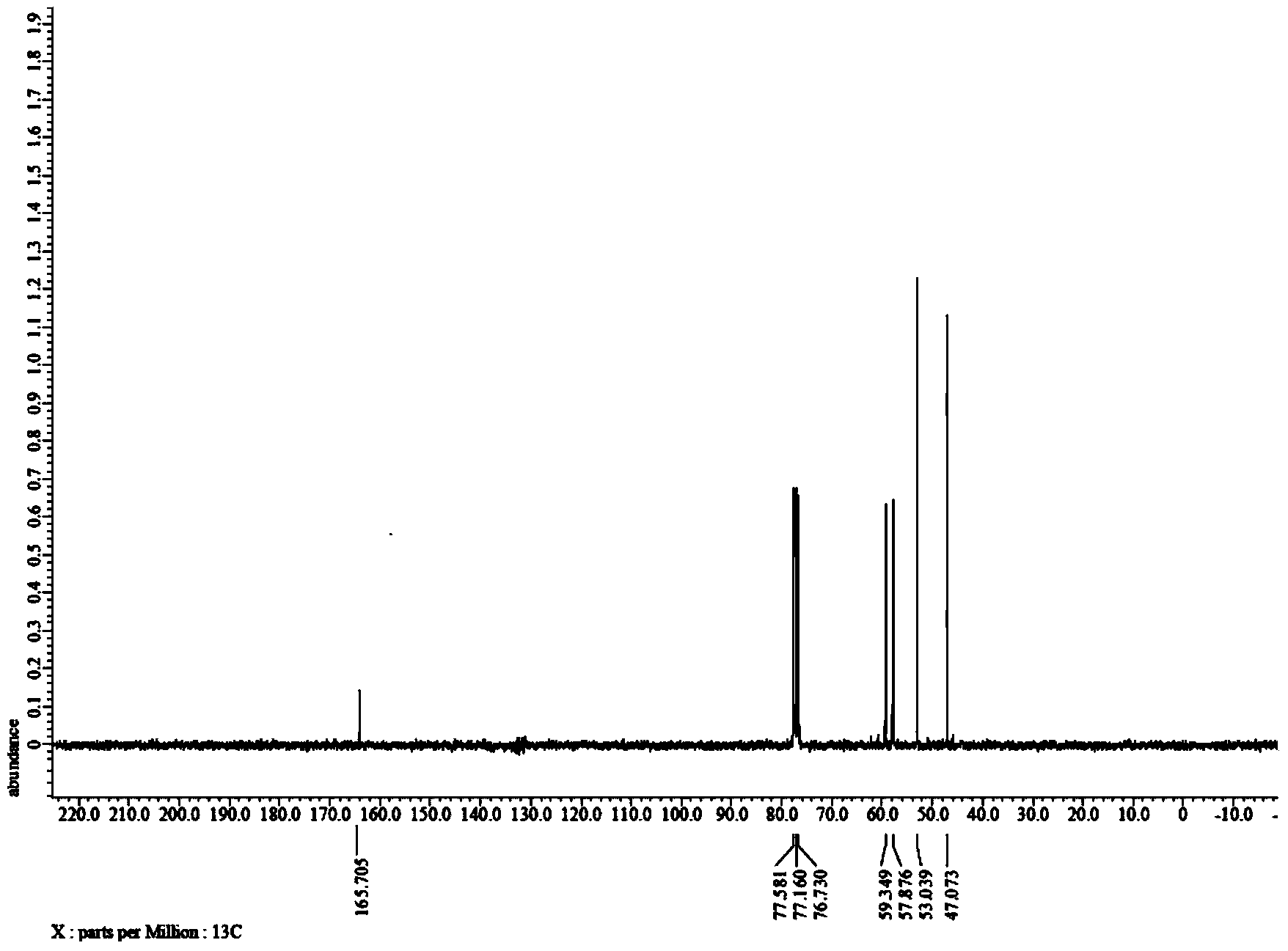
Method for preparing 3-(2-hydroxyethyl)-2-oxazolidone
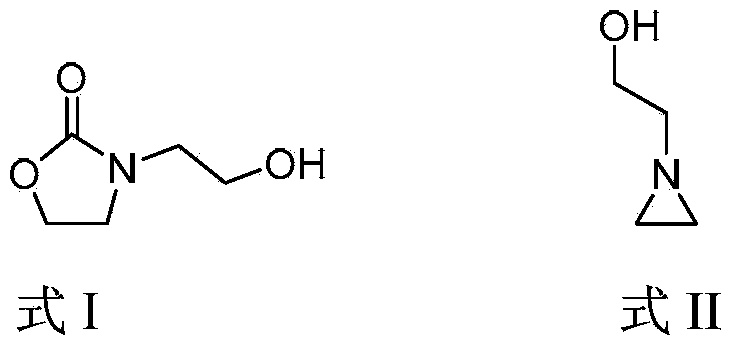
ActiveCN103965129AReduce manufacturing costHigh activityOrganic chemistryChemical recyclingMetal catalystOxazolidone
The invention discloses a method for preparing 3-(2-hydroxyethyl)-2-oxazolidone. The method comprises a step of by taking a nitrogen-containing compound as a catalyst, carrying out a cyclization reaction on a compound 1-(2-hydroxyethyl) ethylenimine shown in formula II in atmosphere of carbon dioxide to obtain the compound 3-(2-hydroxyethyl)-2-oxazolidone shown in formula I. By using non-metal nitrogen-containing organic molecules as a catalyst system, the method disclosed by the invention effectively avoids pollution of metal ions on finished oxazolidone due to use of a metal catalyst, and is cheap and easily available; the adopted catalyst, which is stable non-metal organic molecules, can be recycled, and the catalyst system is environmentally friendly.
Owner:TSINGHUA UNIV
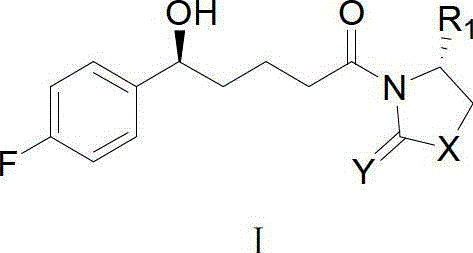
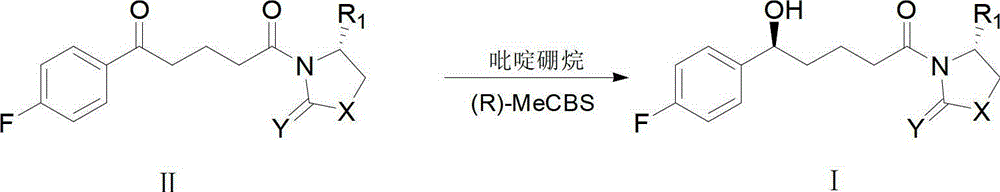
Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone
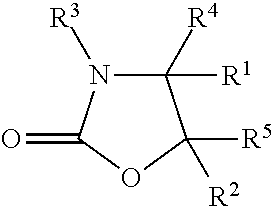
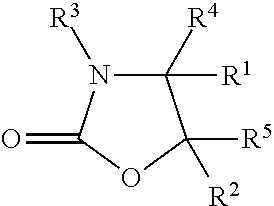
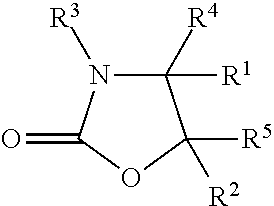
The invention provides a method for synthesizing an important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone. The method comprises the following steps of: 1, enabling fluorobenzoicacid butanoic acid and (S)-4-phenyl-2 oxazolidone to react under the action of a condensing agent to generate a compound (I); and 2, reducing the compound (I) through a chiral reducing agent so as to obtain a compound (II).
Owner:北京京卫燕康药物研究所有限公司
Synthetic method for ezetimibe intermediate
The invention discloses a synthetic method for an ezetimibe intermediate. The synthetic method comprises: by taking a compound I as a raw material, mixing the compound I with a reaction solution; under the action of an acid-binding agent, firstly activating the compound I by pivaloyl chloride; then coupling the compound with S-4-phenyl-2-oxazolidinone; then carrying out reduction reaction through (R)-2-mehtyl-CBS-oxazole borane; and then carrying out post-treatment to prepare (4S)-3-[(5S)-5-(4-fluorophenyl-5-hydroxyl valeryl)-4-phenyl-1,3- azacyclocyclopentane-2-(one) (II), wherein the formula is as shown in the description, and the reaction solution comprises tetrahydrofuran, chloroform, dioxane or dichloromethane. The synthetic method for the ezetimibe intermediate disclosed by the invention has the advantages of being simple to operate, short in synthetic line and relatively low in synthetic cost, and is suitable for large-scaled industrial production.
Owner:WUXI FORTUNE PHARMA
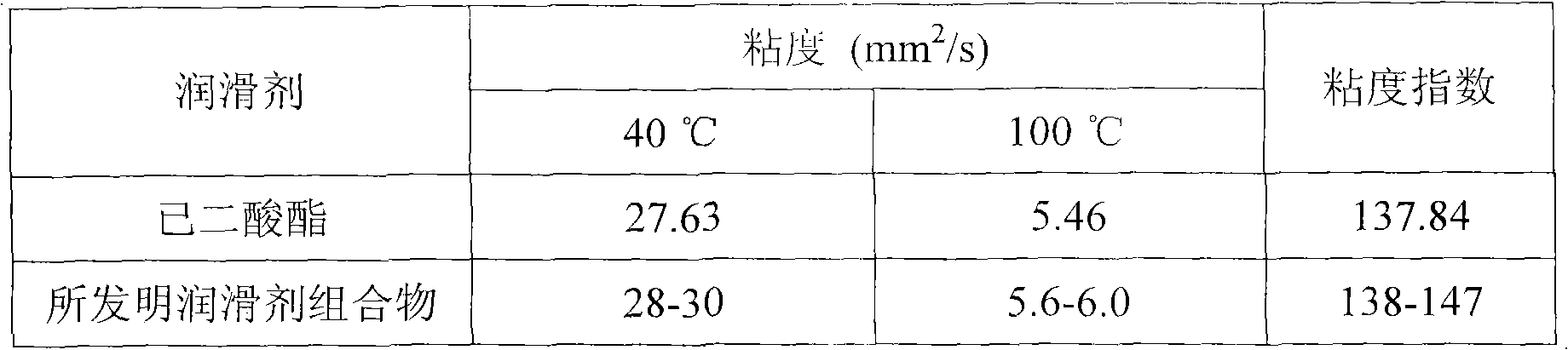
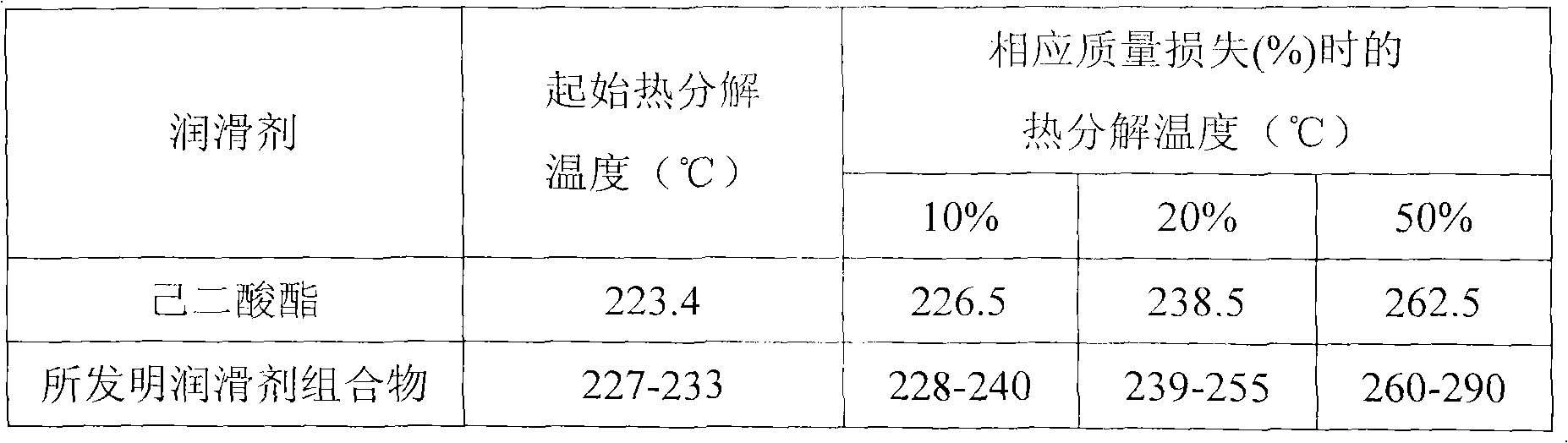
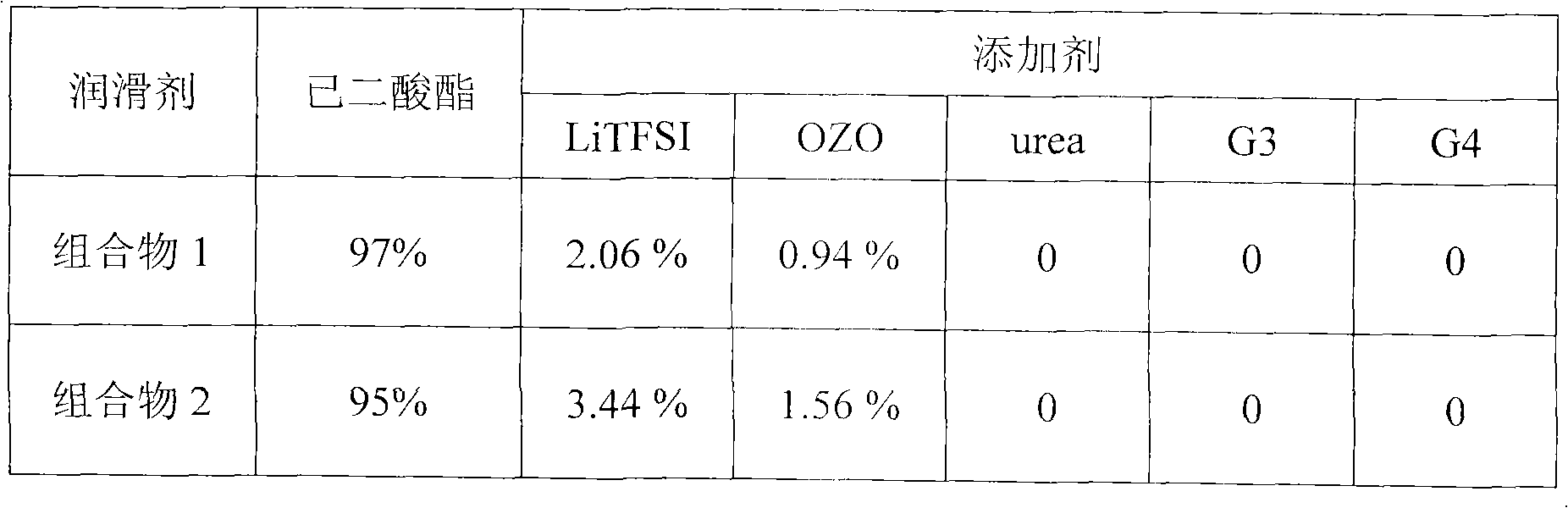
Lubricant composition containing ionic liquid and manufacture method thereof
The invention discloses a lubricant composition containing ionic liquid and a manufacture method of the lubricant composition. The composition comprises base oil and ionic liquid, wherein the mass fraction of the base oil is 99%-95% and the mass fraction of the ionic liquid is 1%-5%. The base oil is adipate and the ionic liquid is a mixture compounded by one or two selected from bistrifluoromethanesulfonimide lithium and 2-Oxazolidone or urea or triethyle ne glycol dimethyl ether or tetraethylene glycol dimethyl ether. The ionic liquid and the lubricant composition can be synchronously compounded. The ionic liquid is simple in process, low in raw material cost and suitable for industrialization and has good chemical stability and thermal stability. The lubricant composition has good anti-wear performance and abrasion resistance to steel or a steel friction pair.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Mono (hydroxyalkyl) urea and holoside cross-linked system
A composition containing a poly-functional molecule; a polysaccharide having a molecular weight of at least 10,000; and mono(hydroxyalkyl)urea and / or 2-oxazolidone crosslinking agents is disclosed. The poly-functional molecule contains at least two functional groups selected from the group consisting of carboxyl, anhydride and amine. The composition is useful as a crosslinking system.
Owner:AKZO NOBEL NV
Method for preparing sofosbuvir intermediate
The invention provides a method for preparing a sofosbuvir intermediate. The method includes following steps: allowing 2-C-methyl-4, S-O-(1-methylethylene)-D-arabonic acid ethyl cyclosulfate and 4-phenyl-2-oxazolidone to be in condensation reaction to obtain a first compound; allowing the first compound and a fluorinating agent for fluorinating reaction, and adding acid for deprotection reaction to obtain a second compound; allowing the second compound to react with sodium ethoxide to obtain a third compound; allowing the third compound to be in rearrangement reaction, and allowing a rearrangement product to react with benzoyl chloride to obtain the sofosbuvir intermediate. The method provides a new idea for synthesizing the sofosbuvir intermediate.
Owner:湖南千金湘江药业股份有限公司
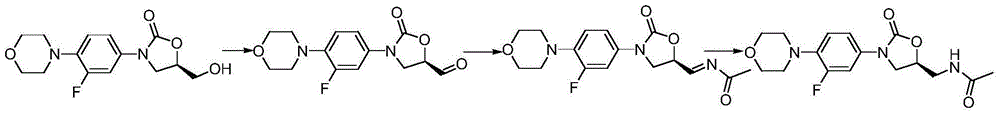
Linezolid preparation method
The invention discloses a linezolid preparation method. Dichloromethane is taken as a solvent, a potassium bromide solution is added, cooling is carried out, (5R)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-hydroxymethyl-2-oxazolidinone is added, a catalyst of a tetramethyl piperidinyloxyl nitride oxide is added while stirring is carried out, a sodium hypochlorite solution is added dropwise, after adding is carried out dropwise, reaction is carried out for 1-3 hours at the temperature of 0-10 DEG C, dichloromethane is added for extraction, an organic phase is dried and filtered through anhydrous sodium sulfate, and (5R)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-formaldehyde-2-oxazolidinone is obtained after the solvent is condensed. Methyl alcohol is taken as a solvent, the (5R)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-formaldehyde-2-oxazolidinone is added and heated to 20-50 DEG C, acetamide is slowly added, liquid-phase chromatogram track reaction is conducted, a reduction agent is directly added after the reaction is completed, precipitation and filtering are carried out, and linezolid is obtained. The method is moderate in reaction condition and environmentally friendly, and can be used for industrial large-scale production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Cyclization method of 4-substituted-2-oxazolidone
The invention discloses a cyclization method of 4-substituted-2-oxazolidone. The method is implemented through the following steps: (1) acylation: acylating chiral alkamine in a toluene solvent by adopting chloro-formate in the presence of potassium carbonate, so as to obtain chiral alkamine acylate; (2) cyclization: carrying out cyclization reaction through taking PEG-400 as a phase transfer catalyst, thereby obtaining 4-substituted-2-oxazolidone. Compared with the prior art, the cyclization method disclosed by the invention has the advantages that the adopted phase transfer catalyst is low in cost and is free of risk, reacting steps are reduced, the cost is reduced, and the conditions are mild, so that the method is suitable for industrial production.
Owner:NANTONG WANNIANCHANG PHARMA
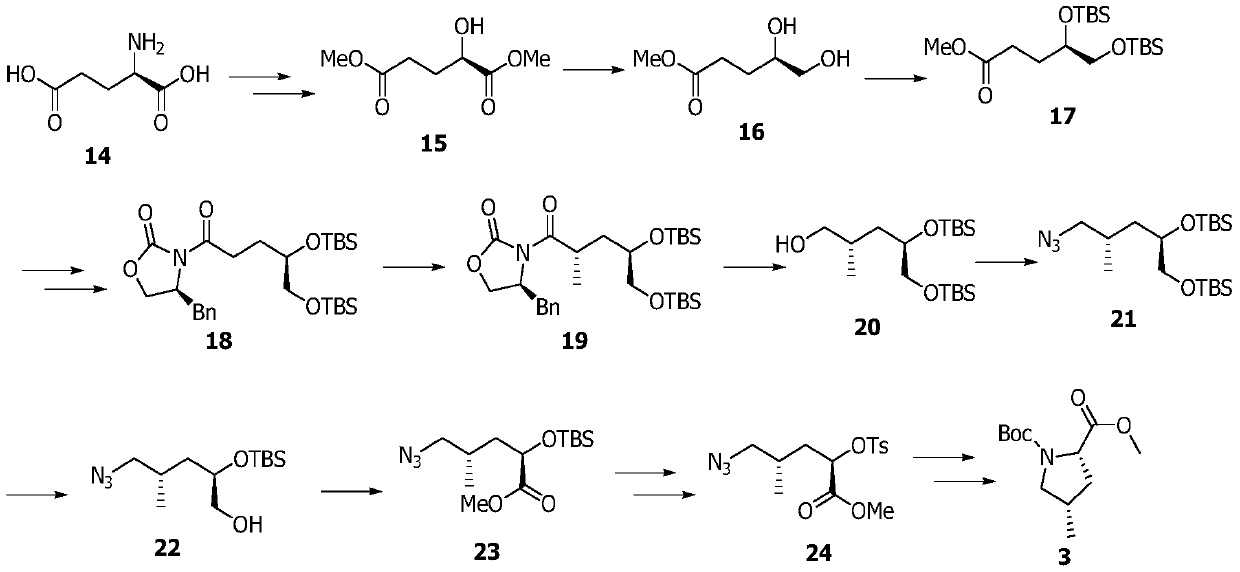
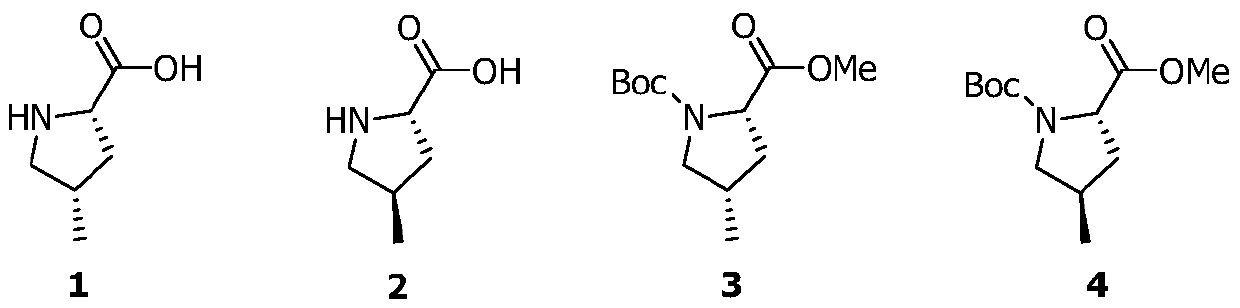
Preparation method of medicinal intermediate N-Boc-cis-4-methyl-L-proline methyl ester
InactiveCN110143906AHigh stereoselectivityHigh yieldOrganic chemistry methodsPotassium hydroxideD-glutamic acid
The invention discloses a preparation method of a medicinal intermediate N-Boc-cis-4-methyl-L-proline methyl ester. The preparation method comprises the following steps: a compound D-glutamic acid isdiazotized, esterified and hydrolyzed to obtain a compound 16; the compound 16 is reacted with TBSCl to obtain a compound 17; the compound 17 is hydrolyzed, and then reacted with (S)-4-benzyl-2-oxazolidone to obtain a compound 18; the compound 18 undergoes a substitution reaction to obtain a compound 19; the compound 19 is reduced to form a compound 20, and the compound 20 is reacted with DPPA andDBU to obtain a compound 21; the compound 21 is deprotected to obtain a compound 22; the compound 22 is dehydroxylated, then is dissolved in methanol, and the obtained solution is reacted in the presence of potassium hydroxide and iodine to obtain a compound 23; the compound 23 is deprotected, and then reacts with paratoluensulfonyl chloride to obtain a compound 24; and the compound 24 undergoesa cyclization reaction under a catalytic condition, an alkaline substance is added, and the obtained mixture reacts with Boc anhydride to obtain a compound 3. The preparation method of the invention overcomes the defect of poor stereoselectivity in the preparation of the N-Boc-cis-4-methyl-L-proline methyl ester in the prior art, uses the cheap and easily-available starting material, and increasesthe yield of the preparation.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +1
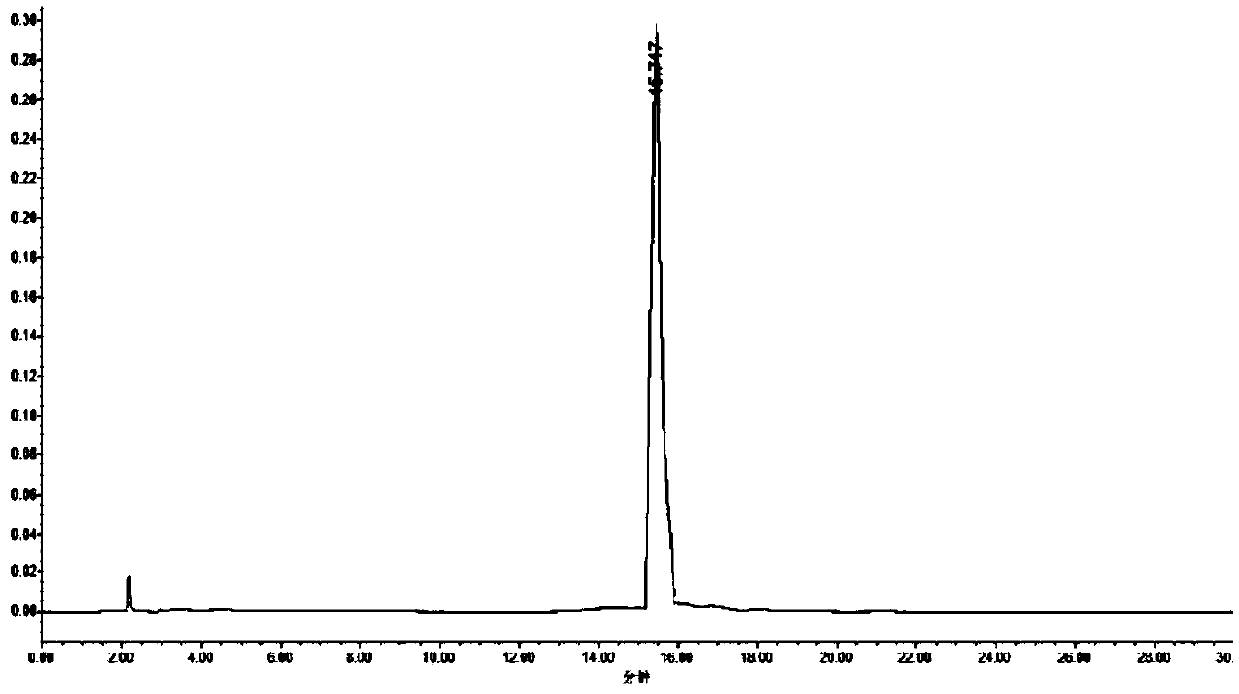
Preparation method of acid hydrolytic impurity in linezolid
The invention discloses a preparation method of an acid hydrolytic impurity in linezolid and belongs to the technical field of medicines. The preparation method comprises the following steps: (1) hydrolysis; (2) distillation: steaming an alcohol organic solvent in the step (1); (3) alkalization and extraction purification; (4) crystallization purification. With the adoption of the method disclosedby the invention, the prepared acid hydrolytic impurity in the linezolid is stable in fine quality and high in purity, is up to 99.942 percent and can be taken as a reference substance of linezolid acid hydrolytic impurity (S)-5-(amino methyl)-3-(3-fluorine-4-morpholine phenyl)-2-oxazolidone, and the reference substance is used for detecting the content of the acid hydrolytic impurity in the linezolid and is of great significance in the quality control of the linezolid.
Owner:GUILIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
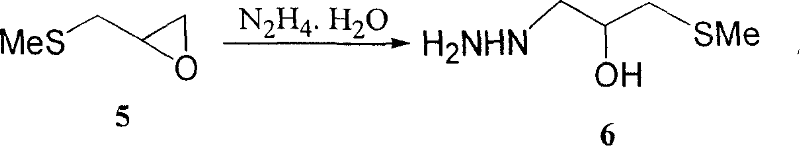
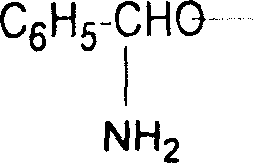
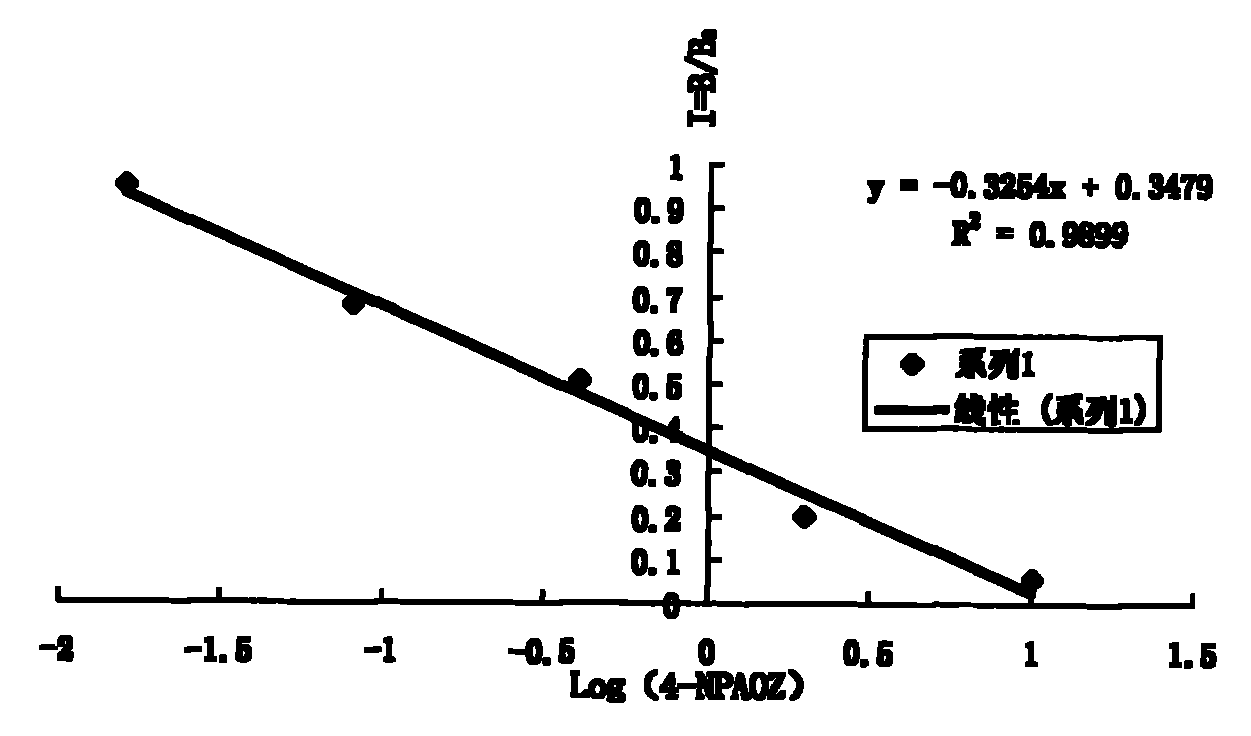

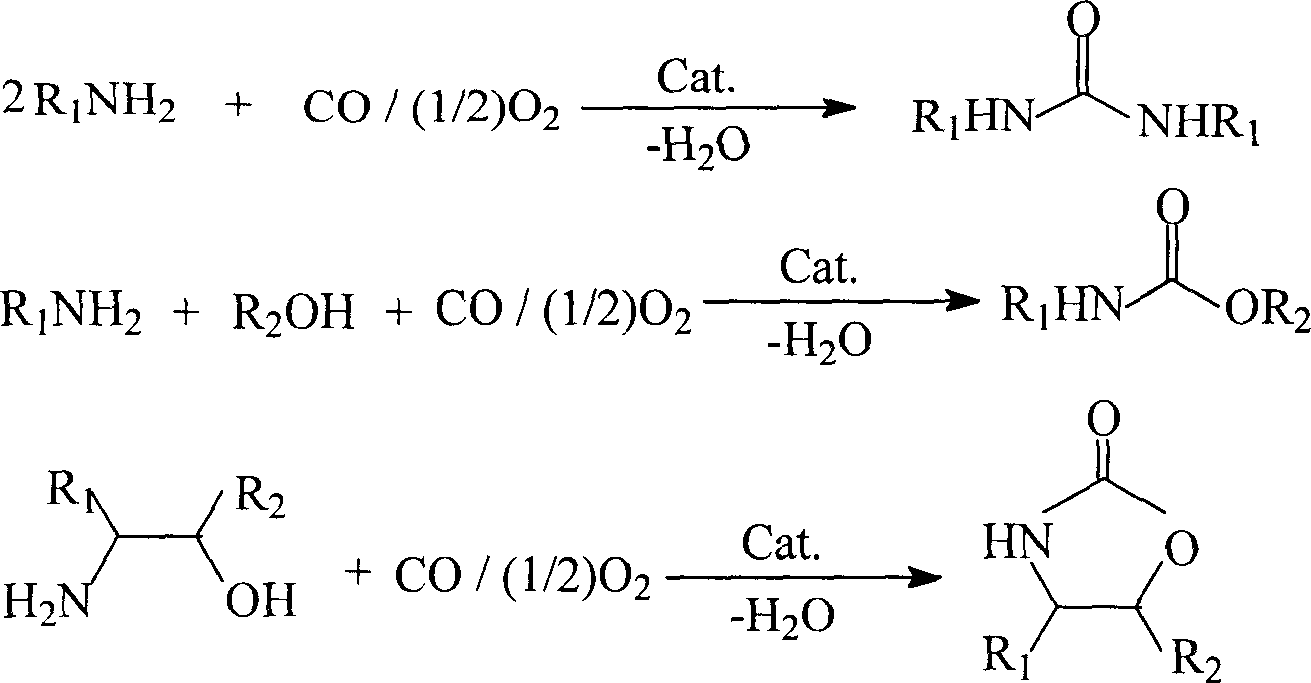
![Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone](https://images-eureka.patsnap.com/patent_img/2253c9ad-04bc-4112-afef-fc155fb5ad42/BSA00000643758300011.PNG)
![Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone](https://images-eureka.patsnap.com/patent_img/2253c9ad-04bc-4112-afef-fc155fb5ad42/BSA00000643758300021.PNG)
![Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone Method for synthesizing important ezetimibe intermediate-(4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-oxazolidine-2-ketone](https://images-eureka.patsnap.com/patent_img/2253c9ad-04bc-4112-afef-fc155fb5ad42/BSA00000643758300031.PNG)