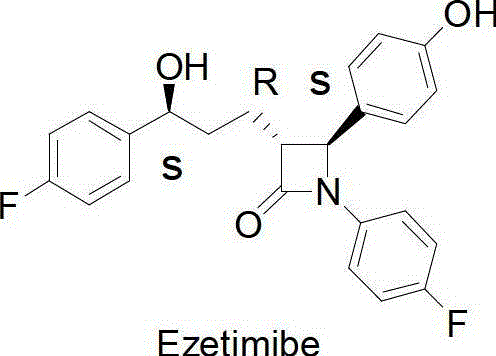
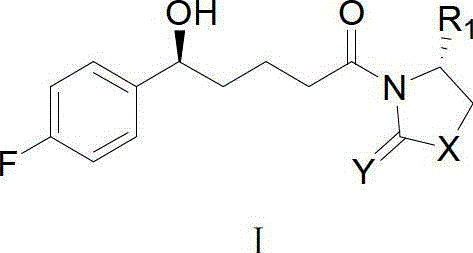
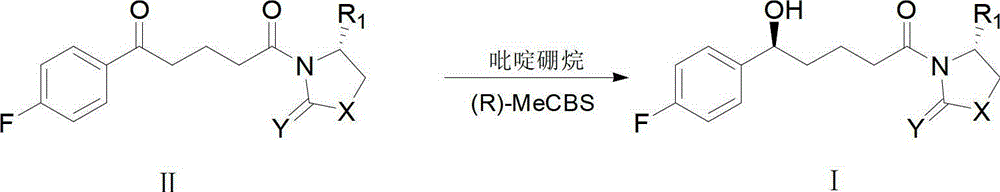
Method for synthesising ezetimibe intermediate
A synthesis method and a technology for etimibe, which are applied in the field of synthesizing etimibe intermediates, can solve the problems of high production cost and complex synthesis process, and achieve the effects of low cost, high optical purity and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. In a dry three-neck flask under nitrogen protection, add 1ml of tetrahydrofuran and 1ml of pH6.5, 0.1M phosphate buffer to form a two-phase mixed solution, the pH of the two-phase mixed solution is 5.5.
[0043] 2. Add 1g of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone into the three-necked flask, 0.005g of carbonyl reductase, stirred and reacted in a water bath at 20°C for 30h to obtain (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4 - Phenyl-2-oxazolidinone solution.
[0044] 3. Filter the (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4-phenyl-2-oxazolidinone solution to obtain The filtrate was concentrated in vacuo, washed with dichloromethane, and dried over anhydrous magnesium sulfate to obtain oily (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4 - Pure phenyl-2-oxazolidinone.
[0045] (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4-phenyl-2-oxazolidinone was detected by mass spectrometry, and the mass sp...
Embodiment 2
[0047] 1. Add 15ml of tetrahydrofuran and 1ml of PH6.5 and 0.1M phosphate buffer to a dry three-neck flask under nitrogen protection to form a two-phase mixed solution. The pH of the two-phase mixed solution is 6.5.
[0048] 2. Add 1g of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone into the three-necked flask, 0.001g of carbonyl reductase, stirred and reacted in a water bath at 30°C for 20h to obtain (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4 - Phenyl-2-oxazolidinone solution.
[0049] 3. Filter the (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4-phenyl-2-oxazolidinone solution to obtain The filtrate was concentrated in vacuo, washed with dichloromethane, and dried over anhydrous magnesium sulfate to obtain oily (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4 - Pure phenyl-2-oxazolidinone.
Embodiment 3
[0051] 1. Add 10ml of tetrahydrofuran and 1ml of a mixed solution of pH 6.5 and 0.1M phosphate buffer into a dry three-neck flask under nitrogen protection. The pH of the two-phase mixed solution is 6.0.
[0052] 2. Add 1g of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone into the three-necked flask, 0.01g of carbonyl reductase, stirred and reacted in a water bath at 40°C for 15h to obtain (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4 - Phenyl-2-oxazolidinone solution.
[0053] 3. Filter the (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4-phenyl-2-oxazolidinone solution to obtain The filtrate was concentrated in vacuo, washed with dichloromethane, and dried over anhydrous magnesium sulfate to obtain oily (4S)-3-[(5S)-(4-fluorophenyl)-5-hydroxyl-1-oxopentyl]-4 - Pure phenyl-2-oxazolidinone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com