Preparation method for nifuratel
A technology of nifuratel, molar ratio, applied in the field of preparation of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
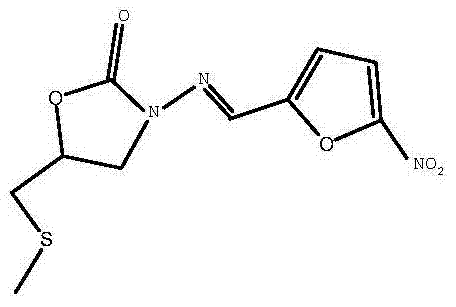
[0041] (5) Preparation of Nifuratel (Target Compound 1)
[0042] In an ice-water bath, add the mixture obtained in step (3) dropwise to the reaction system obtained in step (4), and stir at 0-40°C for 1-4 hours, typically at room temperature for about 1 hour, then let it stand 2 hours. During this period, the target product 1 was produced as a yellow precipitate, which could be obtained by filtration. The crude product was purified by recrystallization. In this step, after the two reaction systems are mixed, sodium ethoxide and phosphoric acid are neutralized, and the operation of removing acid and alkali respectively is omitted.
[0043]Different from the prior art using acetic acid for recrystallization, the present invention uses N,N-dimethylformamide or 1,4-dioxane for recrystallization. This is beneficial to the stability of nifuratel. Nifuratel is a Schiff base, which will be partially decomposed under high temperature and acidic conditions. The mass ratio of nifura...
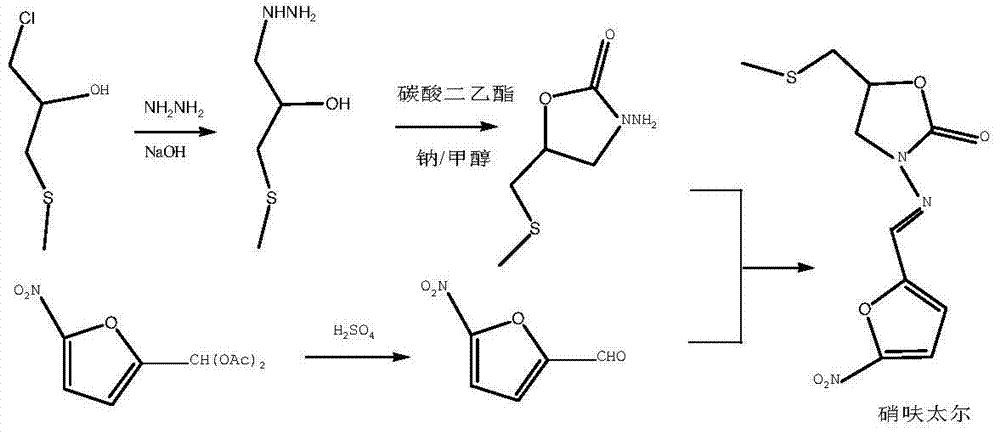
Embodiment 1
[0046] Add 297.9 grams (3.22mol) of epichlorohydrin and 35.5 grams (0.161mol) of 15-crown-5 into the reaction flask, stir mechanically, add dropwise 1127 grams (content: 20%, 3.22mol) of sodium methyl mercaptide, React at 20°C for 4 hours, a white solid is produced in the reaction bottle, add water to dissolve the solid, separate the liquids, wash the organic phase with water, dry over anhydrous magnesium sulfate, and filter with suction to obtain 330 g (3.17 mol) of intermediate 3 with a purity of 98.5% , yield 98.4%.
[0047] Take 990 g (15.85 mol) of hydrazine hydrate and add it to the reaction flask, raise the temperature to 95°C, and add the compound intermediate intermediate 3 dropwise under stirring. After the dropwise addition, continue to react at this temperature for 3 hours, cool to room temperature, and then evaporate Water and unreacted hydrazine hydrate yielded 418 g (3.07 mol) of hydrazinolyzed intermediate 4.
[0048] Add 362 grams (3.07 mol) of diethyl carbon...
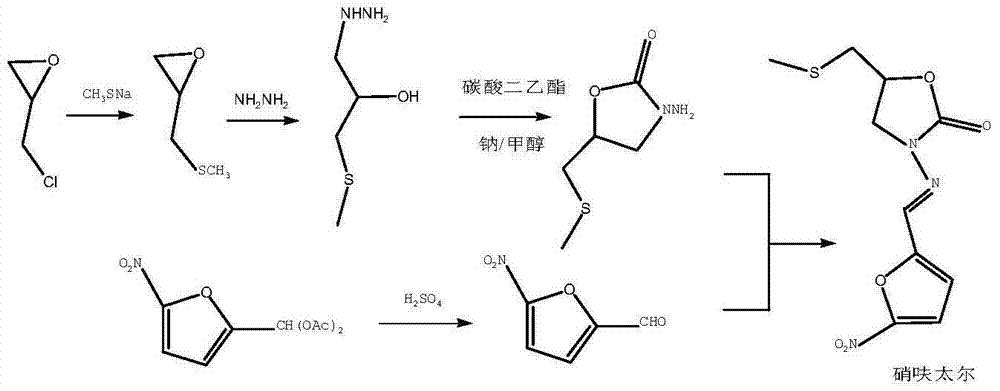
Embodiment 2
[0054] Add 297.9 grams (3.22mol) of epichlorohydrin and 35.5 grams (0.161mol) of 15-crown-5 into the reaction flask, stir mechanically, add dropwise 1127 grams (content: 20%, 3.22mol) of sodium methyl mercaptide, After reacting at 20°C for 4 hours, a white solid was produced in the reaction bottle. Water was added to dissolve the solid, and the liquid was separated. The organic phase was washed with water, dried with anhydrous magnesium sulfate, and filtered with suction to obtain 330 g (3.17 mol) of intermediate 3.
[0055] Take 990 g (15.85 mol) of hydrazine hydrate and add it to the reaction flask, raise the temperature to 95°C, and add the compound intermediate intermediate 3 dropwise under stirring. After the dropwise addition, continue to react at this temperature for 3 hours, cool to room temperature, and then evaporate Water and unreacted hydrazine hydrate yielded 418 g (3.07 mol) of hydrazinolyzed intermediate 4.
[0056] Add 362 grams (3.07 mol) of diethyl carbonate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com