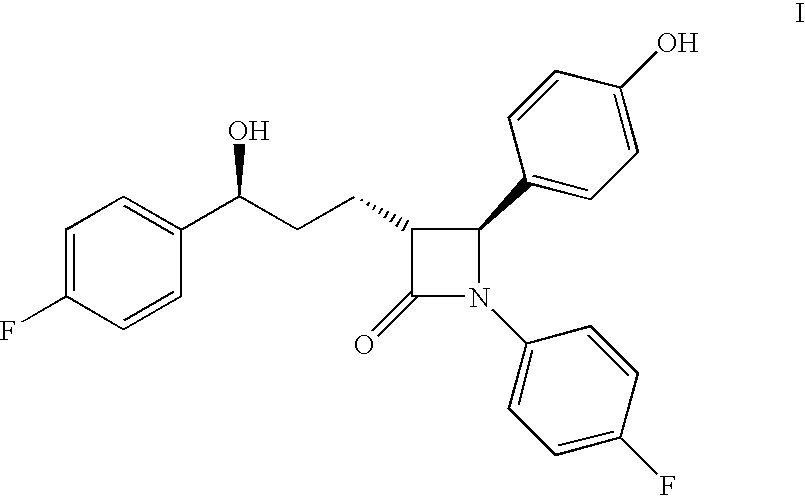
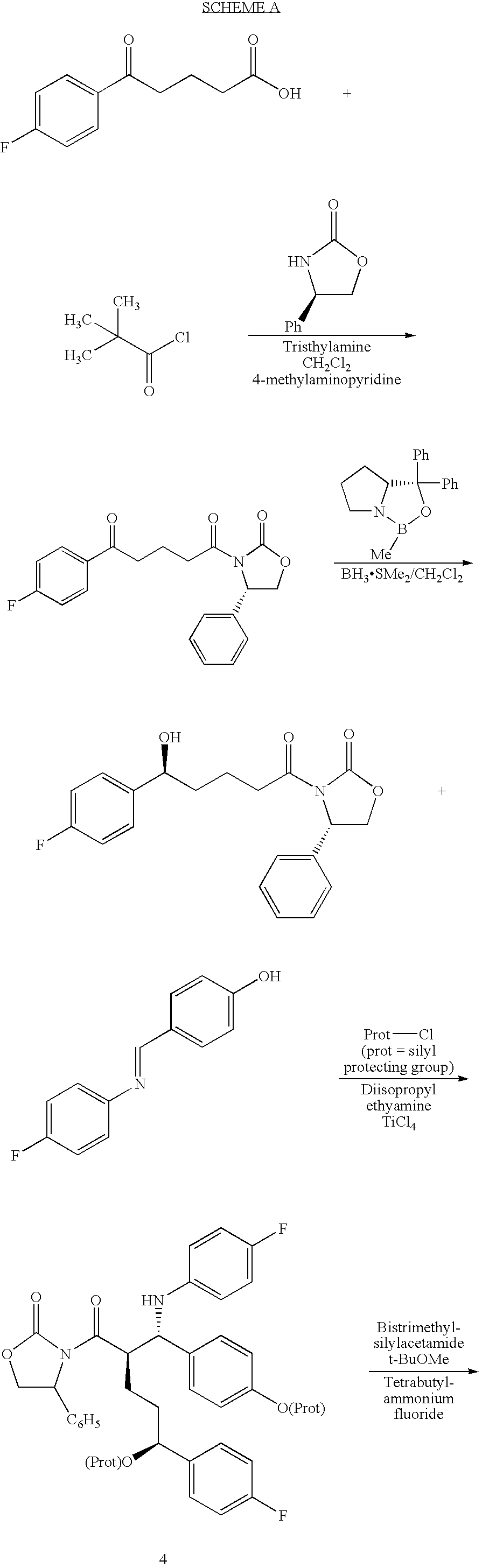
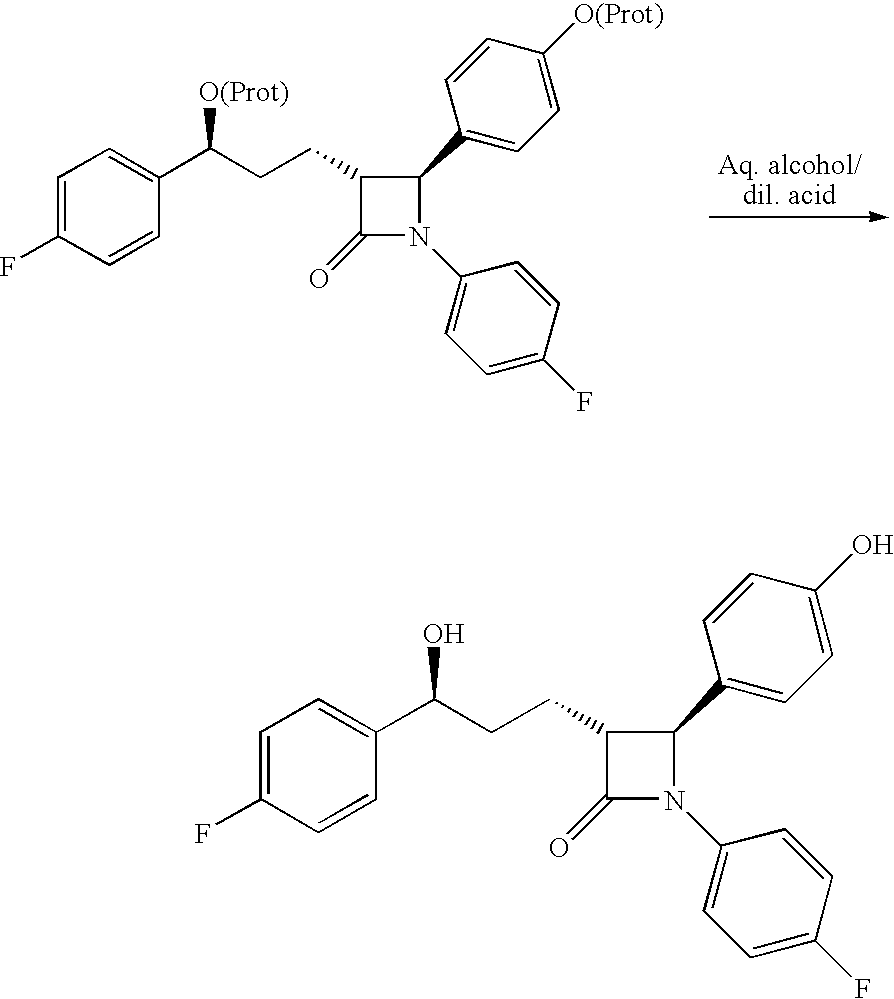
Process for ezetimibe intermediate
a technology of ezetimibe and intermediate, which is applied in the field of process for the preparation of ezetimibe intermediate, can solve the problems of unsuitable industrial production of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone (100 gm) is dissolved in toluene (750 ml), the mixture of (−)-β-chlorodiisopinocampheylborane ((−)-DIP chloride) in heptane (545 ml, 1.5M) and toluene (750 ml) is added at 0° C. to 5° C. for 1 hour. The reaction mixture is stirred for 15 hours at 25° C. to 30° C. and 340 ml of 10% sodium chloride is then added at the same temperature. The layers are separated and the organic layer is washed with 5% sodium bicarbonate (300 ml), 1N sulfuric acid (300 ml), and 10% sodium chloride (300 ml). Then the organic layer is dried on sodium sulfate to give 3-[(5S)-5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-2-oxazolidinone in 96% yield.
example 2
[0031]The organic layer of 3-[(5S)-5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-2-oxazolidinone from example 1 is mixed with 4-fluoro-N-(4-hydroxyphenyl)methylene-benzenamine (121 gm) and cooled to −10° C. Then diisopropylethylamine (260 ml) is added to the reaction mixture for 45 minutes at −10° C. to −15° C., trimethylsilylchloride (135 ml) is added and stirred for 1 hour at −20° C. to −25° C. The reaction mixture is cooled to −30° C., TiCl4 (35 ml) is slowly added to the reaction mixture at −30° C. to −35° C. and stirred for 3 hours at the same temperature. 5% Aq. tartaric acid solution (1700 ml) is added to the reaction mixture at 0° C., stirred for 1 hour and allowed the temperature to rise to 25° C. Then 20% Aq. NaHSO3 (350 ml) solution and stirred for 2 hours at 25° C. to 30° C. The organic layer is separated and washed with 1000 ml water, concentrated to 250 ml volume and added 100 ml bistrimethylsilylacetamide. Then the reaction mixture is heated to reflux for 30 minu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling temperature | aaaaa | aaaaa |
| boiling temperature | aaaaa | aaaaa |
| boiling temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com