Synthetic method of ezetimibe, and intermediate used in synthetic method
一种化合物、苄基的技术,应用在依泽替米贝的合成领域,能够解决成本高、不经济环保等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
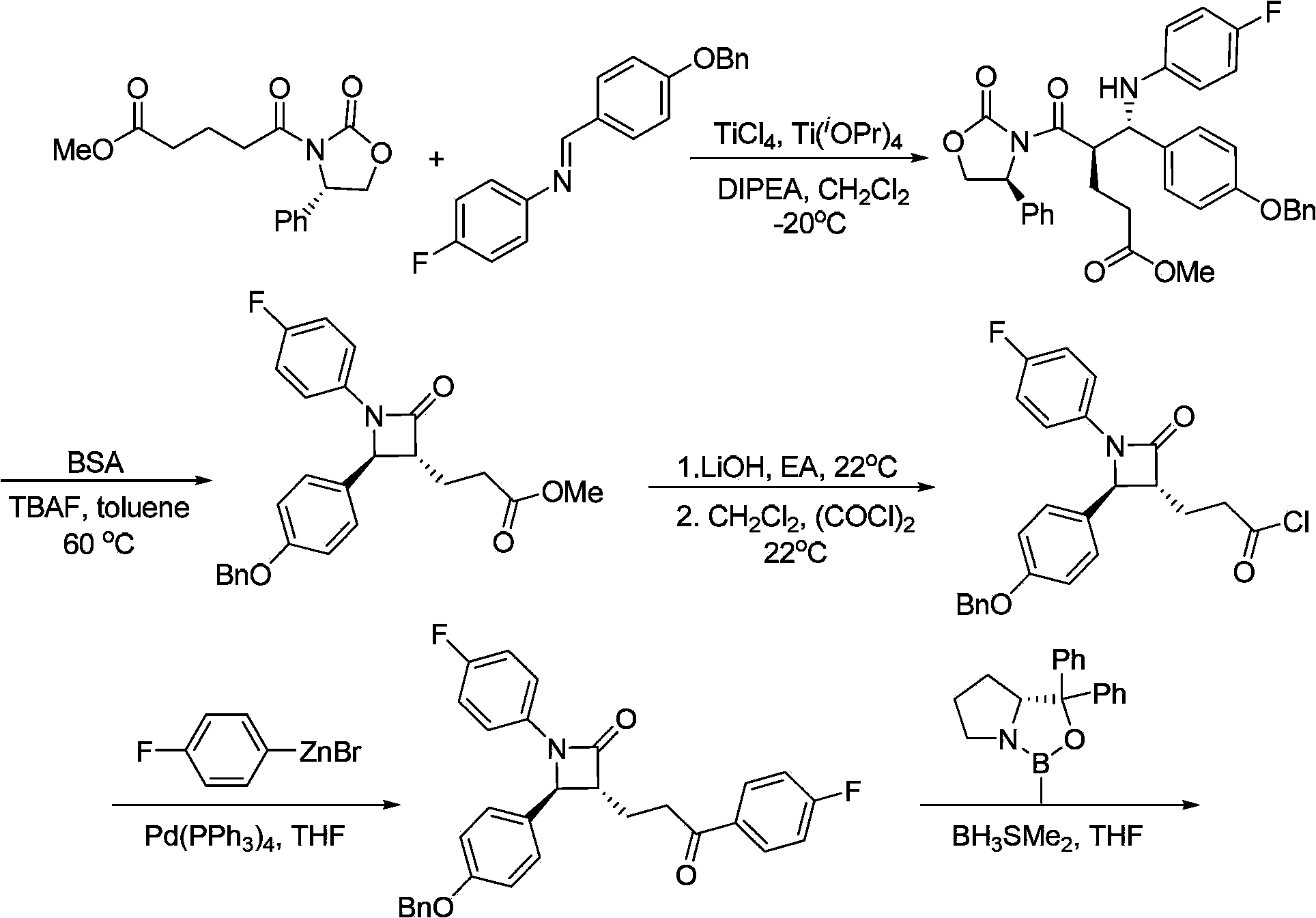
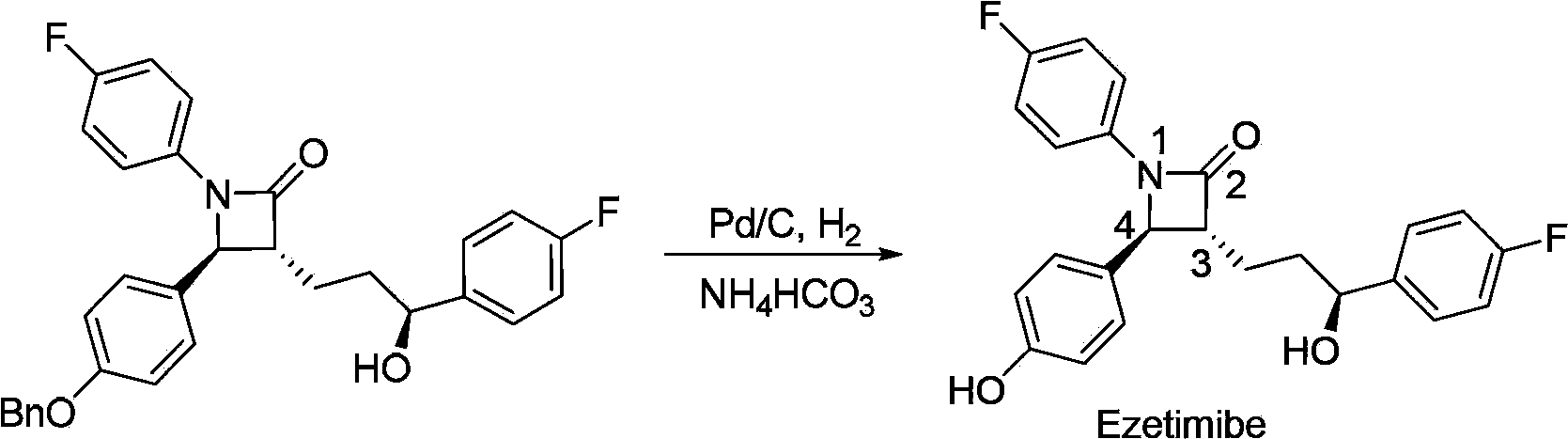
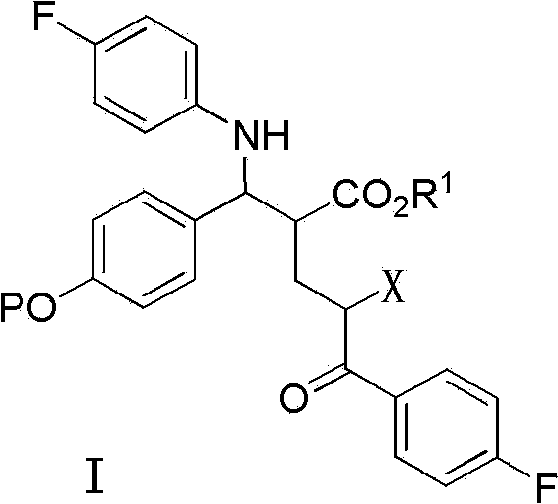
[0129] The preparation method of formula I compound of the present invention comprises the following steps:
[0130] (a) In the presence of a base, the compound of formula 1 and p-fluoroaniline undergoes an allyl amination reaction to prepare the compound of formula 2;
[0131]
[0132] (b) Addition reaction occurs between the compound of formula 2 and the compound of formula 3 under the action of base to prepare the compound of formula I having the structure shown in formula 4A; and optionally,
[0133]
[0134] (c) the compound of formula I having the structure shown in formula 4A removes the ester group at the β-position of the ketone carbonyl to generate the compound of formula I having the structure shown in formula 5A,
[0135]
[0136] Among the various types, P, R 1 , R 2 , The definition of LG is as mentioned above.
[0137] In another preferred example, the step (a) uses a complex formed between a phosphine ligand and a transition metal catalyst precursor...
Embodiment 1
[0197] Example 1 Preparation of compound (R)-2a.
[0198] The reaction is as follows: under argon atmosphere, [Pd(C 3 h 5 )Cl] 2 (54.8mg, 0.15mmol) and (S, S, S)-Lc (193mg, 0.25mmol) were added to a Schlenk tube, and anhydrous CH 2 Cl 2 (50mL), after stirring at room temperature for 10 minutes, successively added substrate 1a (3.54g, 10mmol), K 2 CO 3(1.0M aqueous solution, 30mL, 30mmol) and p-fluoroaniline (3.33g, 30mmol). After stirring at room temperature for three hours, separate the layers, extract the aqueous phase with dichloromethane (3×50 mL), combine the organic phases, dry over anhydrous sodium sulfate, filter and concentrate, and purify by column chromatography to obtain the asymmetric aminated product ( R)-2a. The reaction formula is as follows:
[0199]
[0200] (R)-2a, white solid, 87% yield. Mp72-73°C, [α] D 20 =-111.0(c1.00, CHCl 3 ),95%ee[Determined by high performance liquid chromatography, chiral AD-H column, n-hexane / isopropanol=95:5,1.0mL / mi...
Embodiment 2
[0202] In this embodiment, different phosphine ligands (S, S, S)-L and metal salt [Pd(η-C 3 h 5 )Cl] 2 Catalysts were prepared on site to catalyze the allyl amination reaction of substrate 1a to prepare compound (R)-2a (reaction formula is as follows):
[0203]
[0204] The reaction is as follows: under argon atmosphere, [Pd(C 3 h 5 )Cl] 2 (54.8mg, 0.15mmol) and (S, S, S)-L (0.25mmol) were added to a Schlenk tube, and anhydrous CH 2 Cl 2 (50mL), after stirring at room temperature for 10 minutes, successively added substrate 1a (3.54g, 10mmol), K 2 CO 3 (1.0M aqueous solution, 30mL, 30mmol) and p-fluoroaniline (3.33g, 30mmol). After stirring at room temperature for three hours, separate the layers, extract the aqueous phase with dichloromethane (3×50 mL), combine the organic phases, dry over anhydrous sodium sulfate, filter and concentrate, and purify by column chromatography to obtain the asymmetric aminated product ( R)-2a.
[0205] Table 1: Results of asymmetric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com