Patents
Literature
51 results about "Potassium phthalimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
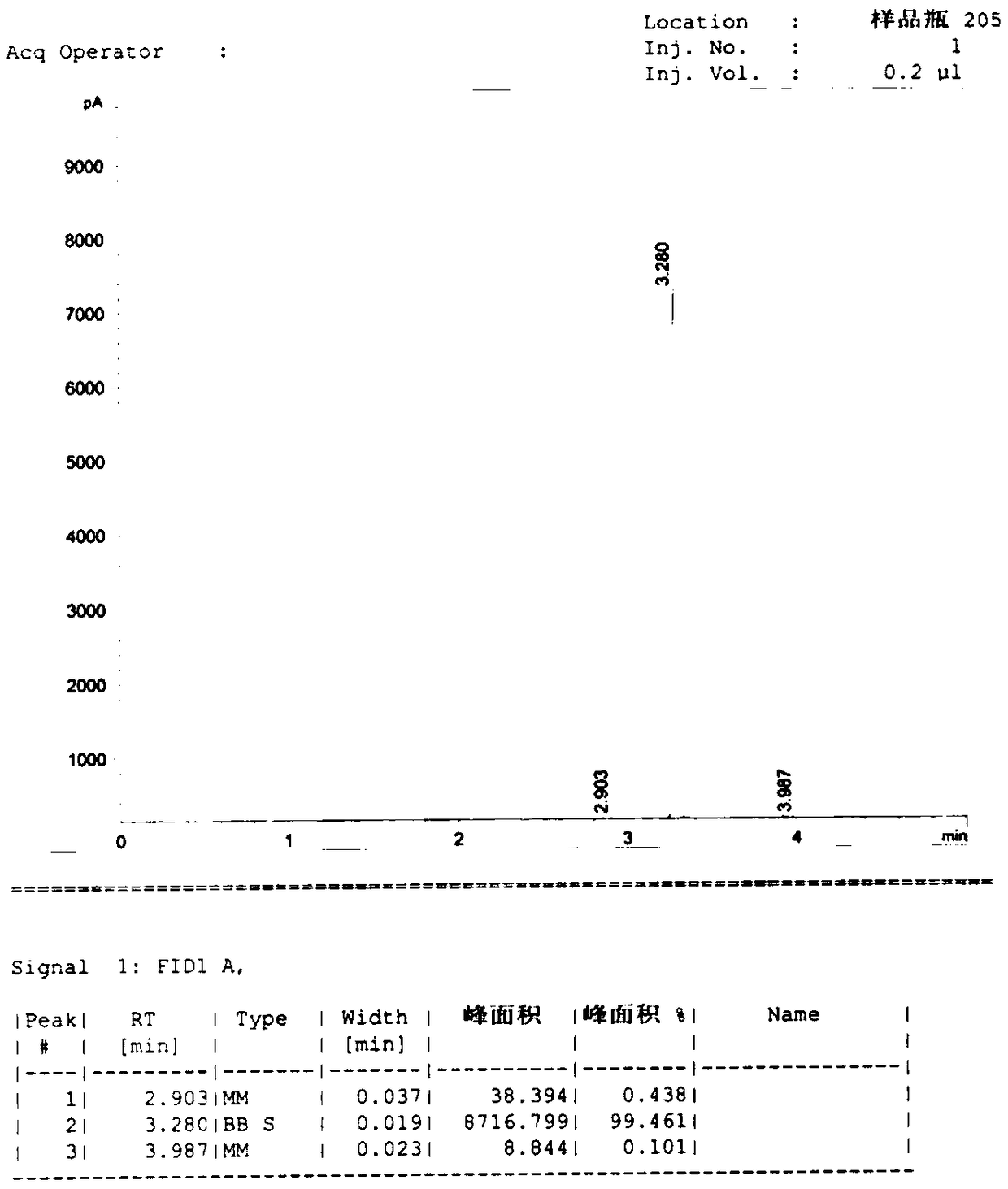
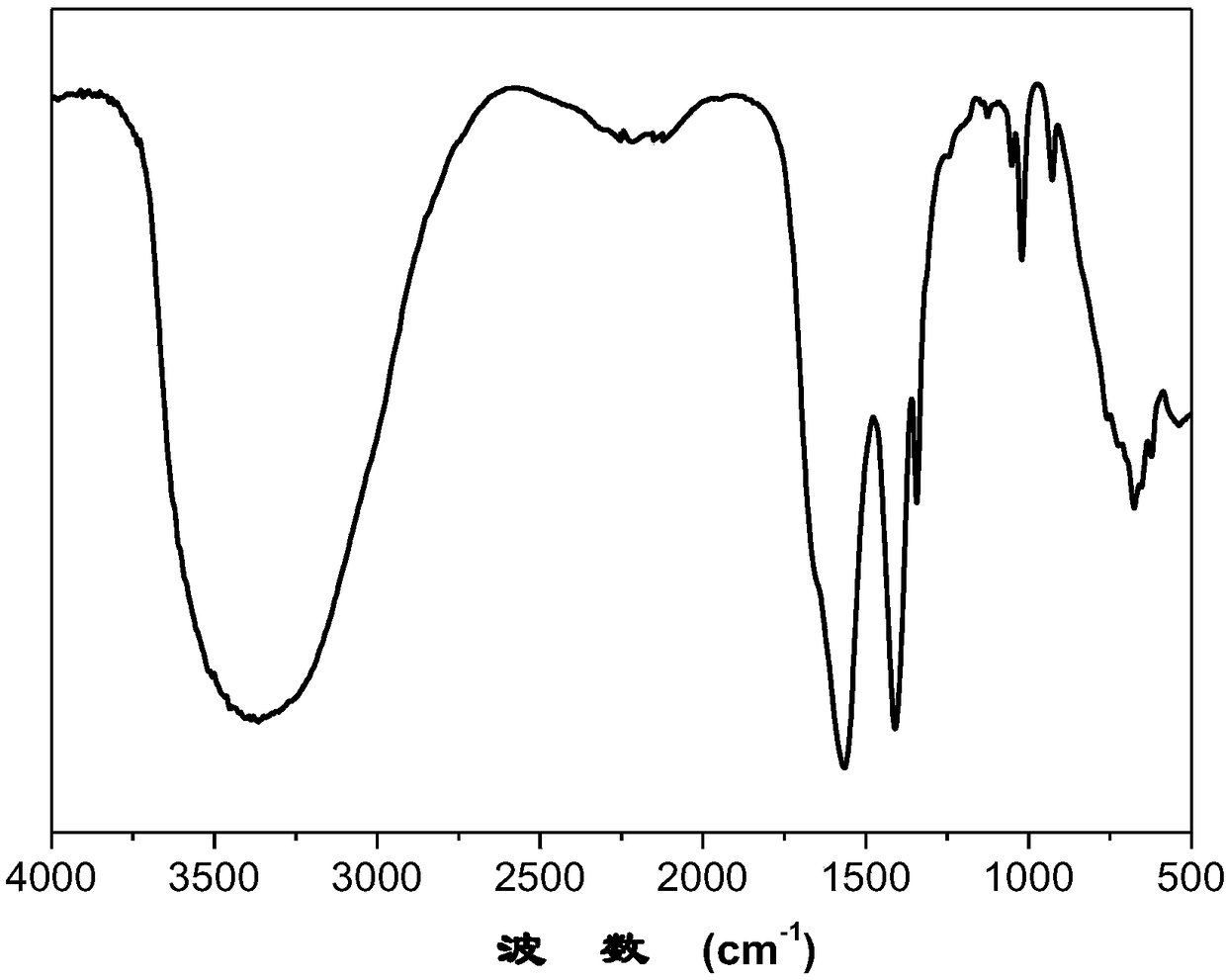
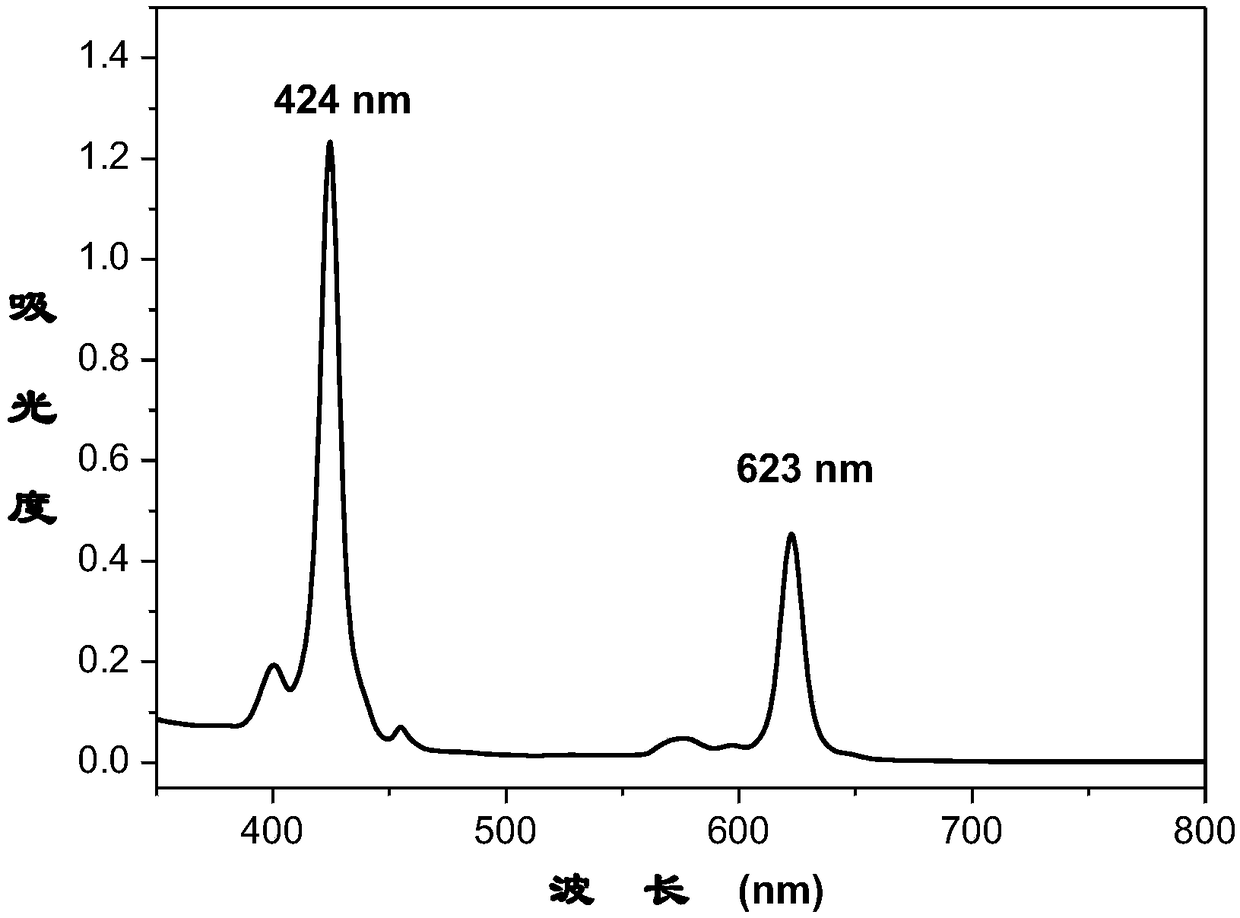
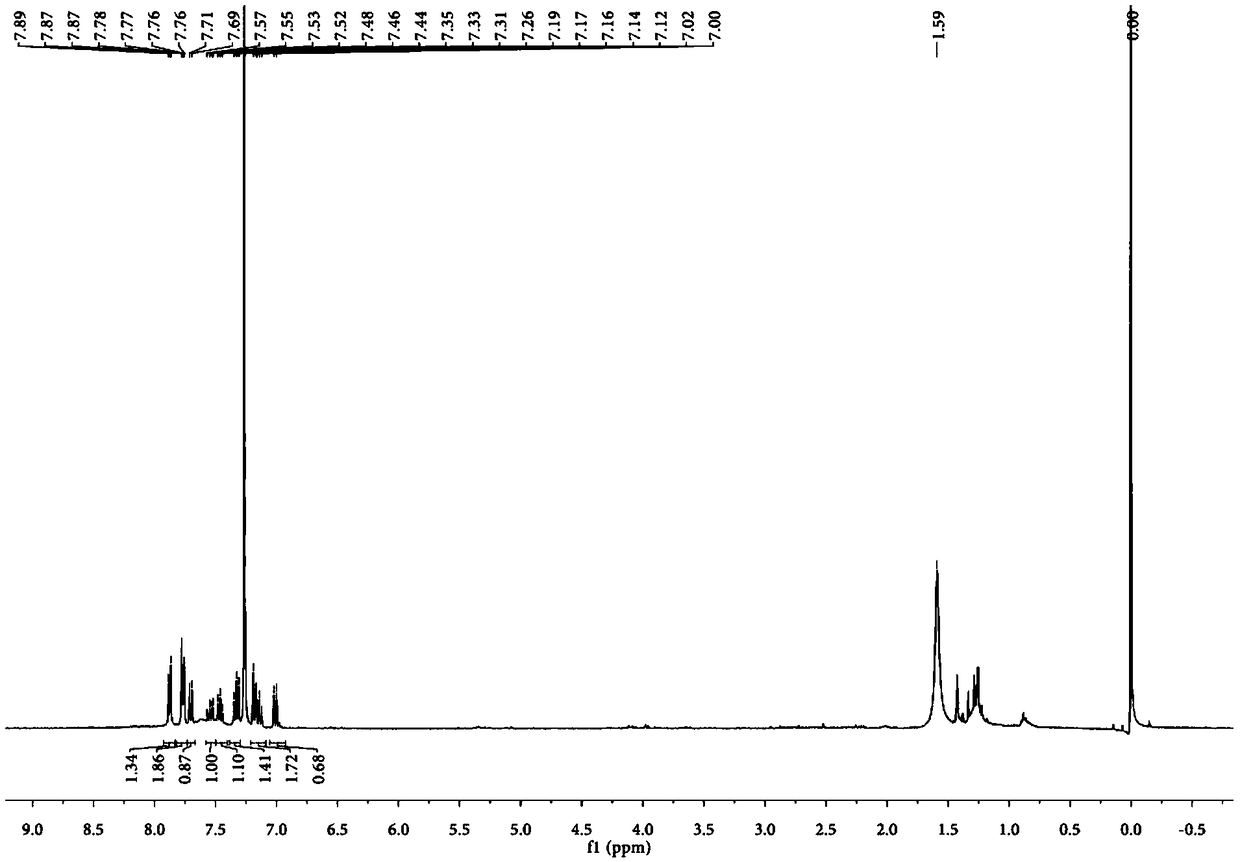
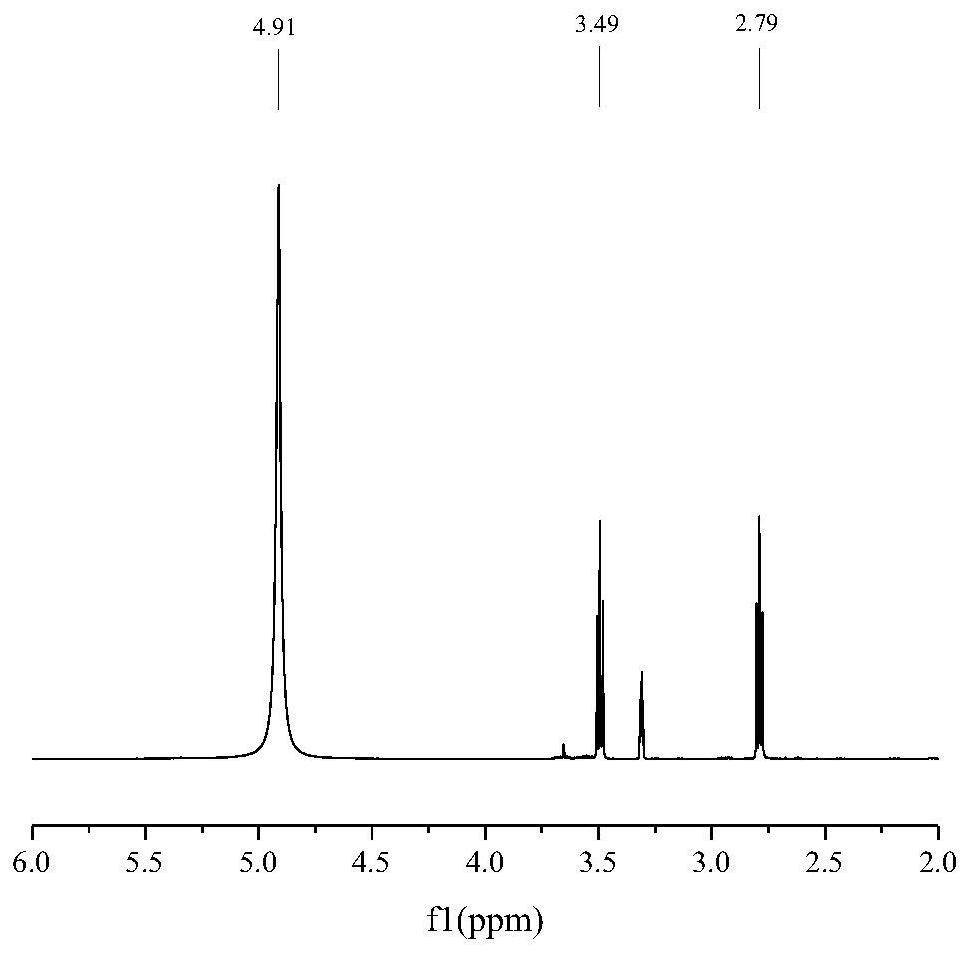
Potassium phthalimide is a chemical compound of formula C₈H₄KNO₂. It is the potassium salt of phthalimide, and usually presents as fluffy, very pale yellow crystals. It can be prepared by adding a hot solution of phthalimide in ethanol to a solution of potassium hydroxide in ethanol; the desired product precipitates.
Novel process for the preparation of linezolid and related compounds
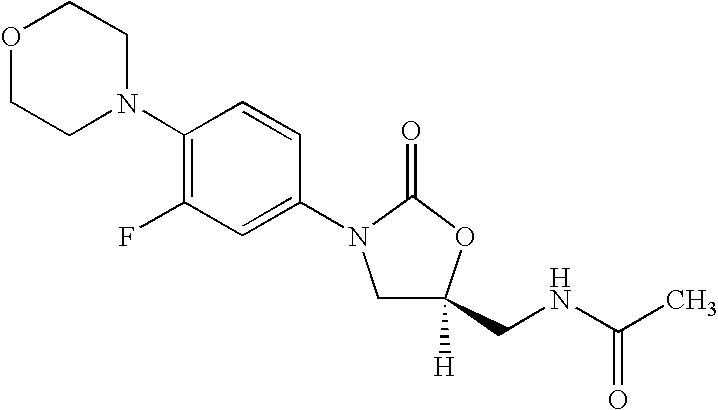
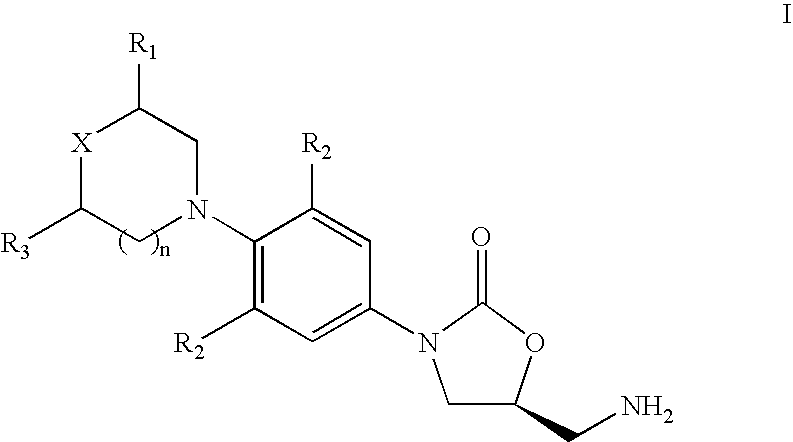
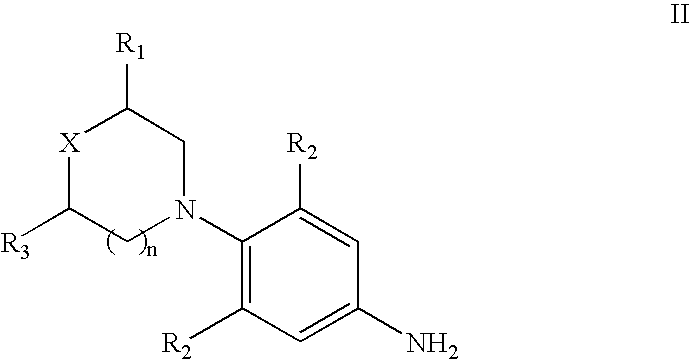
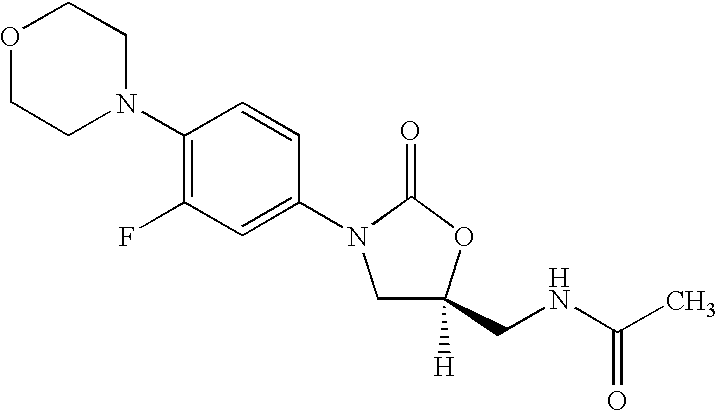
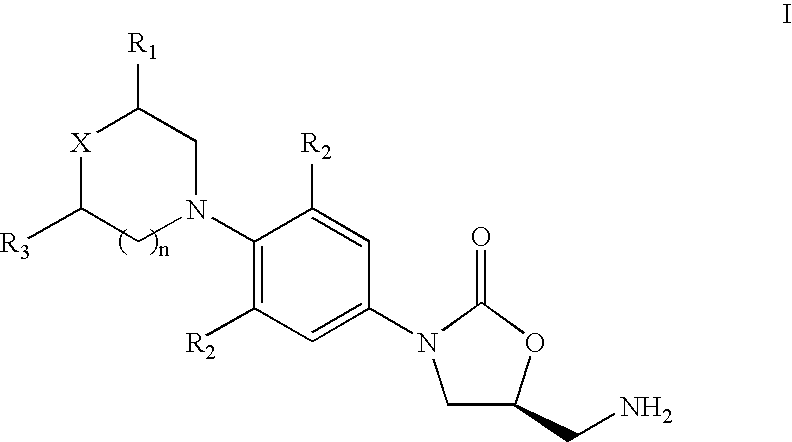
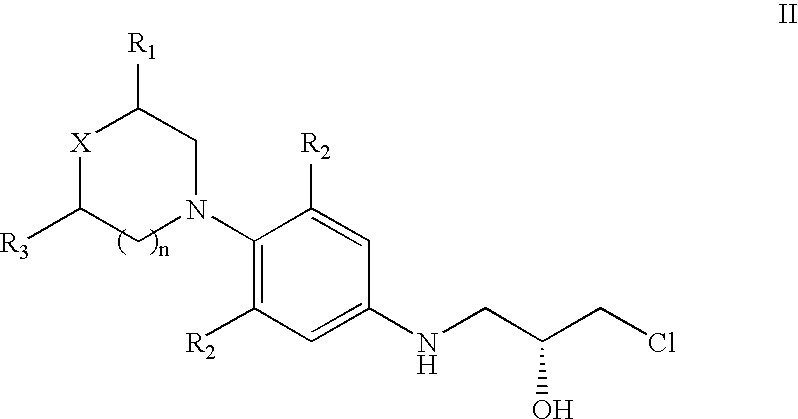
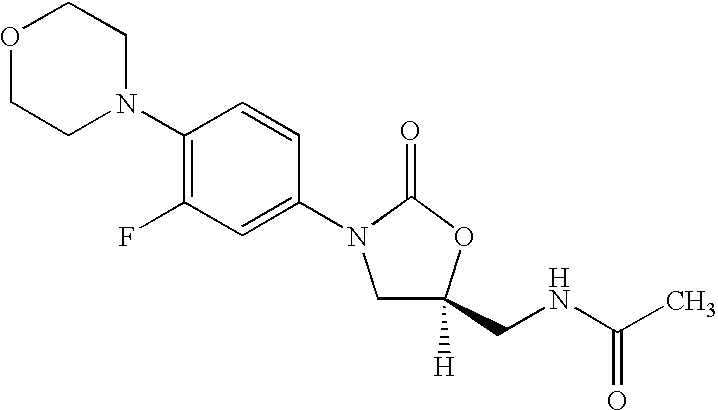
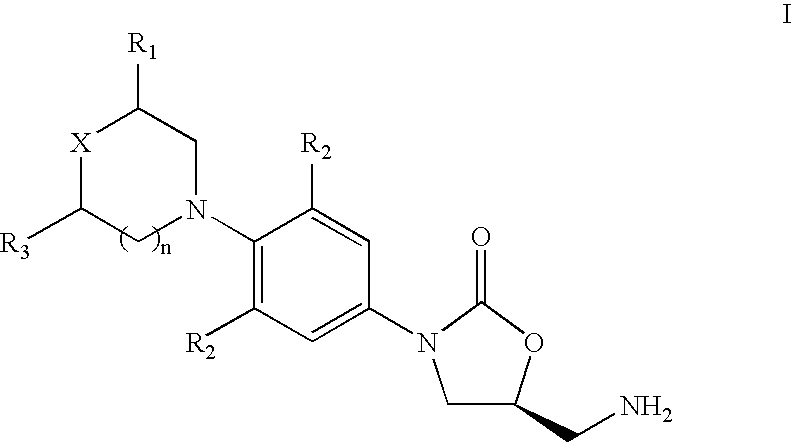
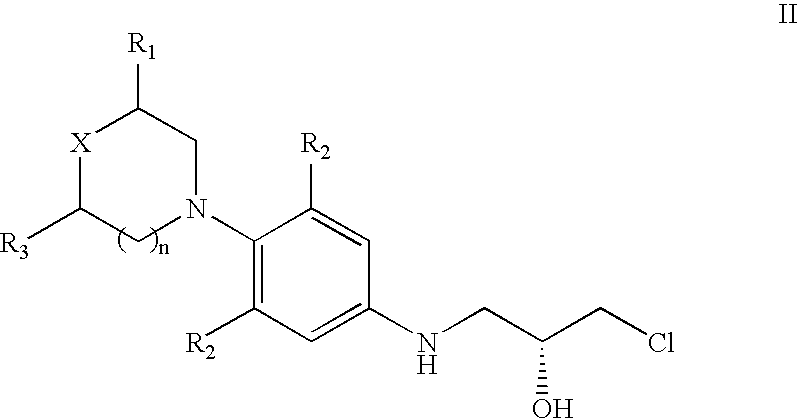
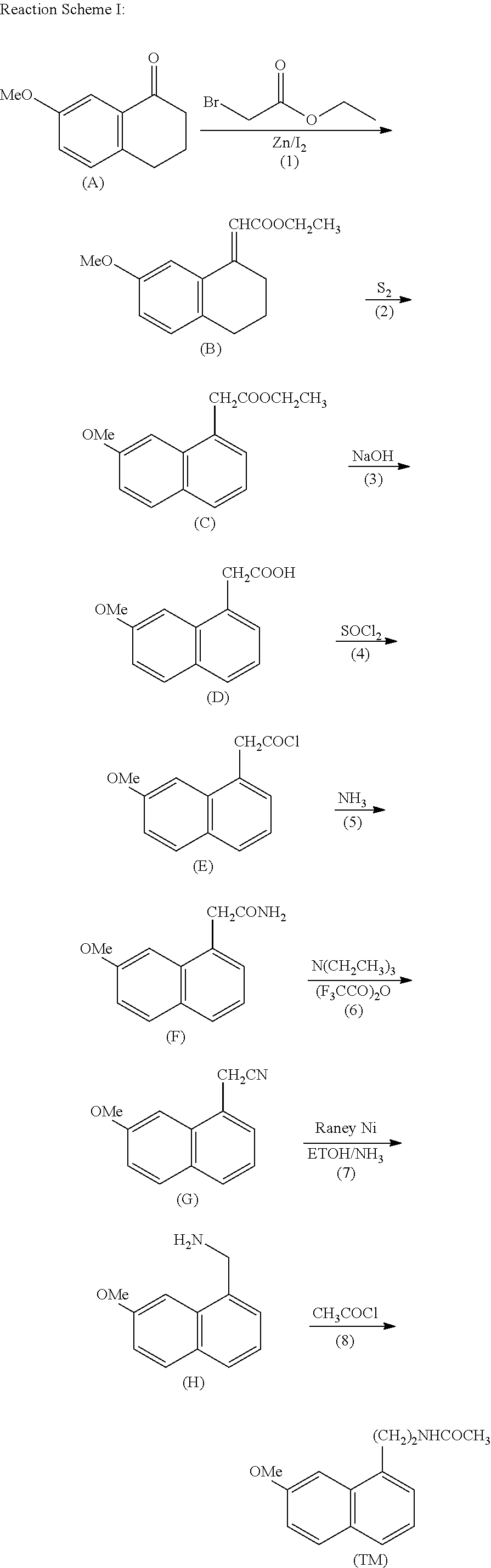
The present invention provides a novel process for preparation of 5-aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus linezolid is prepared by a) reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; b) subjecting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline produced above to carbonylation; c) reacting (5R)-5-(chloromethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone produced above with potassium phthalinide; d) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]phthalimide produced above with hydrazine hydrate; and e) reacting S-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazo-lidinyl]methyl]amine produced above with acetic anhydride to produce linezolid.
Owner:HETERO USA INC
Novel intermediates for linezolid and related compounds
The present invention provides a novel process for preparation of 5 aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus, the key intermediate of linezolid is prepared by a) reacting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline with potassium phthalimide; b) subjecting N-[3-pthalimido-2-(R)-hydroxypropyl]-3-fluoro-4-(morpholinyl) aniline produced in the above step to carbonylation; and c) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidiriyl]methyl]phthalimide produced in the above step with hydrazine hydrate to produce (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]amine.
Owner:HETERO USA INC
Intermediates for linezolid and related compounds
The present invention provides a novel process for preparation of 5 aminomethyl substituted oxazolidinones, key intermediates for oxazolidinone antibacterials including linezolid. Thus, the key intermediate of linezolid is prepared by a) reacting N-[3-Chloro-2-(R)-hydroxypropyl]-3-fluoro-4-morpholinyl aniline with potassium phthalimide; b) subjecting N-[3-pthalimido-2-(R)-hydroxypropyl]-3-fluoro-4-(morpholinyl) aniline produced in the above step to carbonylation; and c) reacting (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidiriyl]methyl]phthalimide produced in the above step with hydrazine hydrate to produce (S)-N-[[3-[3-Fluoro-4-[4-morpholinyl]phenyl]-2-oxo-5-oxazolidinyl]methyl]amine.
Owner:HETERO USA INC
Synthesis method for 5-aminolevulinic acid hydrochloride
InactiveCN102627573AThe synthesis steps are simpleHigh selectivityOrganic compound preparationAmino-carboxyl compound preparationMalonic acidSynthesis methods
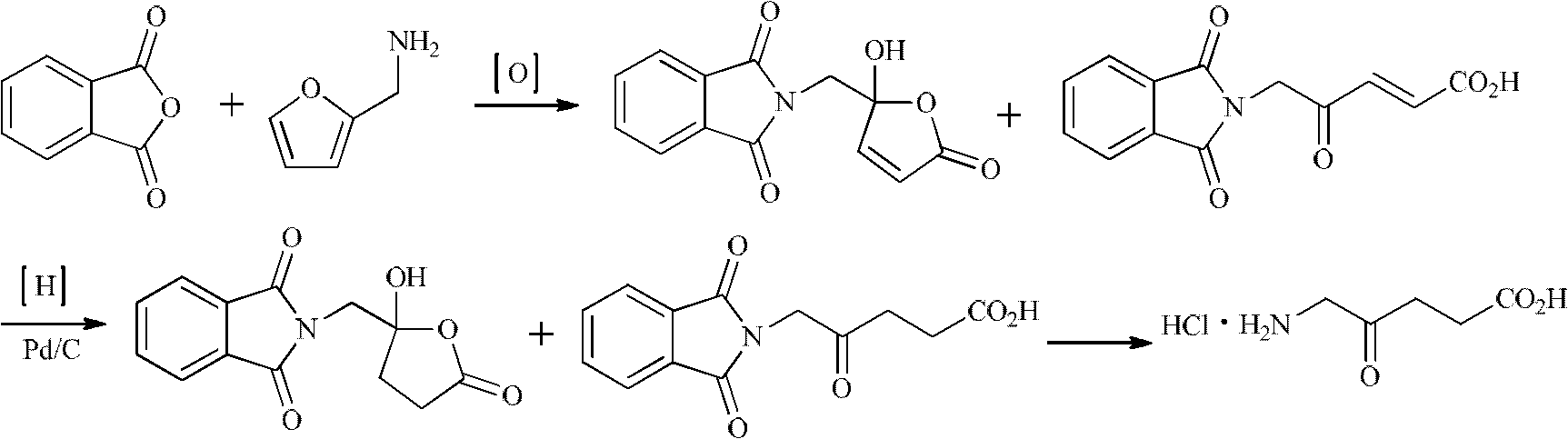
The invention relates to a synthesis method for 5-aminolevulinic acid hydrochloride. The synthesis method comprises the steps as follows: taking 1,3-dichloroacetone as a starting material, carrying out Gabriel reaction on the 1,3-dichloroacetone and phthalimide potassium in a water phase or an organic phase to obtain a high-purity intermediate (I), 2-(3-chloro-2-oxopropyl)isoindoline-1,3-diketone; reacting the intermediate (I) with malonic acid isopropyl ester or malonic acid diethyl ester in alkali condition to prepare an intermediate (II), 2-(3-(2,2-dimethyl-4,6-dioxo-1,3-dioxane-5-yl)-2-oxopropyl)isoindoline-1,3-diketone or an intermediate (III), 2-(3-(1,3-dioxoisobenzazole-2-yl)-2-oxopropyl)malonic acid diethyl ester; and hydrolyzing, decarboxylating and post-processing to obtain the high-purity 5-aminolevulinic acid hydrochloride.
Owner:SHANDONG UNIV
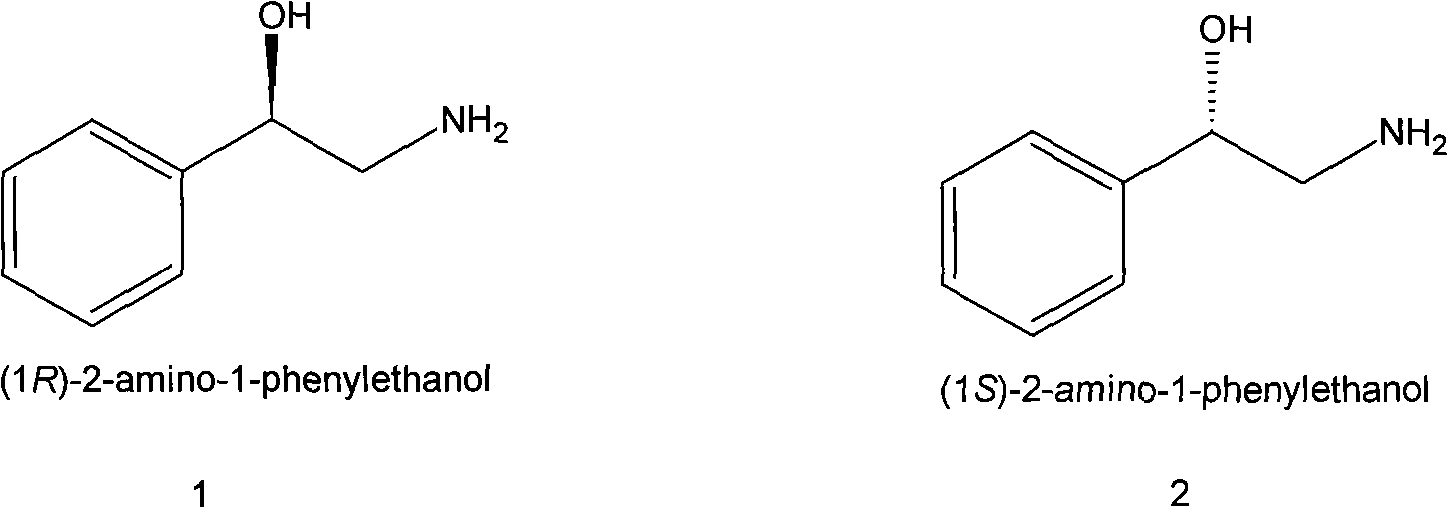
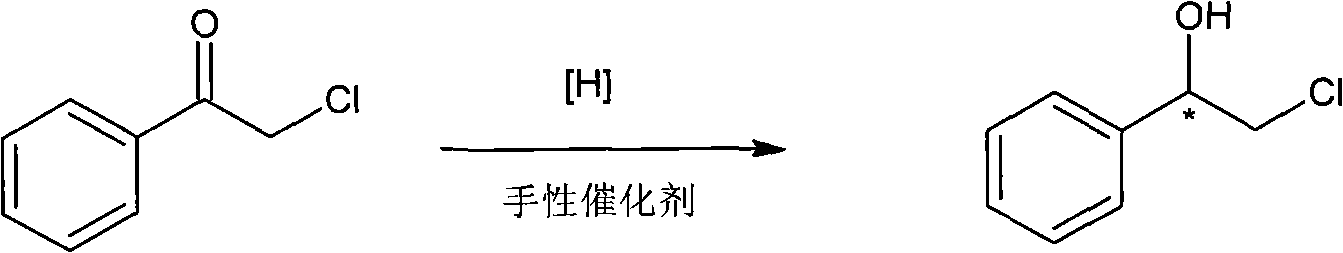
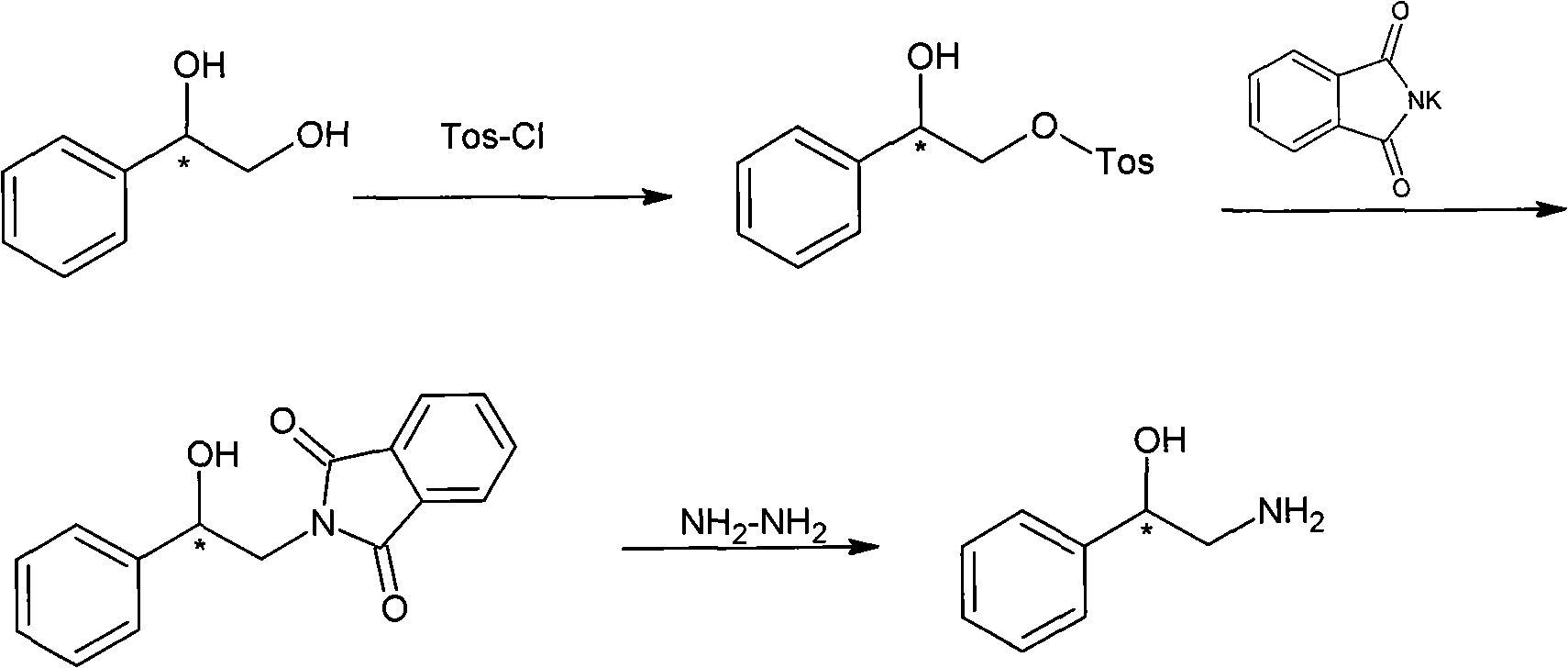
Method for preparing chiral medicinal intermediate 2-amido-1-phenylethylalcohol
ActiveCN101575298AReasonable workmanshipEasy to operateOrganic compound preparationAsymmetric synthesesPotassium phthalimideToluene
The invention relates to a method for preparing a chiral medicinal intermediate 2-amido-1-phenylethylalcohol. The method comprises the following steps that: chiral 1,2-styrolylalcohol is used as a rawmaterial to obtain chiral 2-paratoluenesulfonic-1-phenyl-1,2-glycol through a selective single para toluene sulfonylation reaction; the chiral 2-paratoluenesulfonic-1-phenyl-1,2-glycol performs a substitution reaction with potassium phthalimide to obtain chiral 2-phthalimide-1-phenylethanol; and finally, the chiral medicinal intermediate 2-amido-1-phenylethylalcohol with retained configuration isobtained through a hydrazinolysis reaction. Compared with the prior art, the method has reasonable process and simple operation, prepares the 2-amido-1-phenylethylalcohol from the low-cost chiral rawmaterial with low cost, obtains the product with high optical purity and chemical purity, is suitable for industrial large-scale production, and provides advantageous condition for industrially producing chiral 2-amido-1-phenylethylalcohol analogs.
Owner:上海予利生物科技股份有限公司
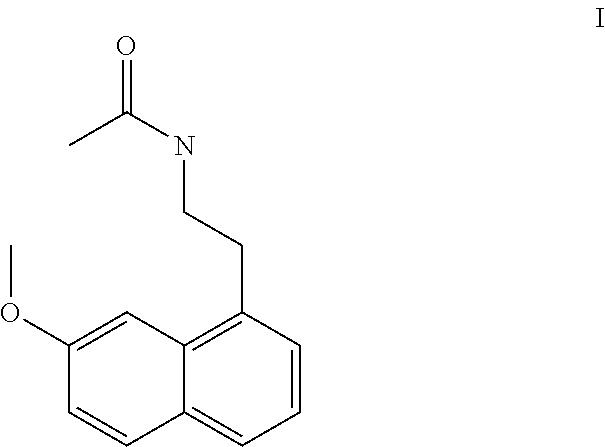
Process for the manufacture of agomelatine and its intermediate
ActiveUS20110130571A1Available and inexpensive materialEasy to optimizeNervous disorderOrganic compound preparationEthyl groupAlkaline hydrolysis
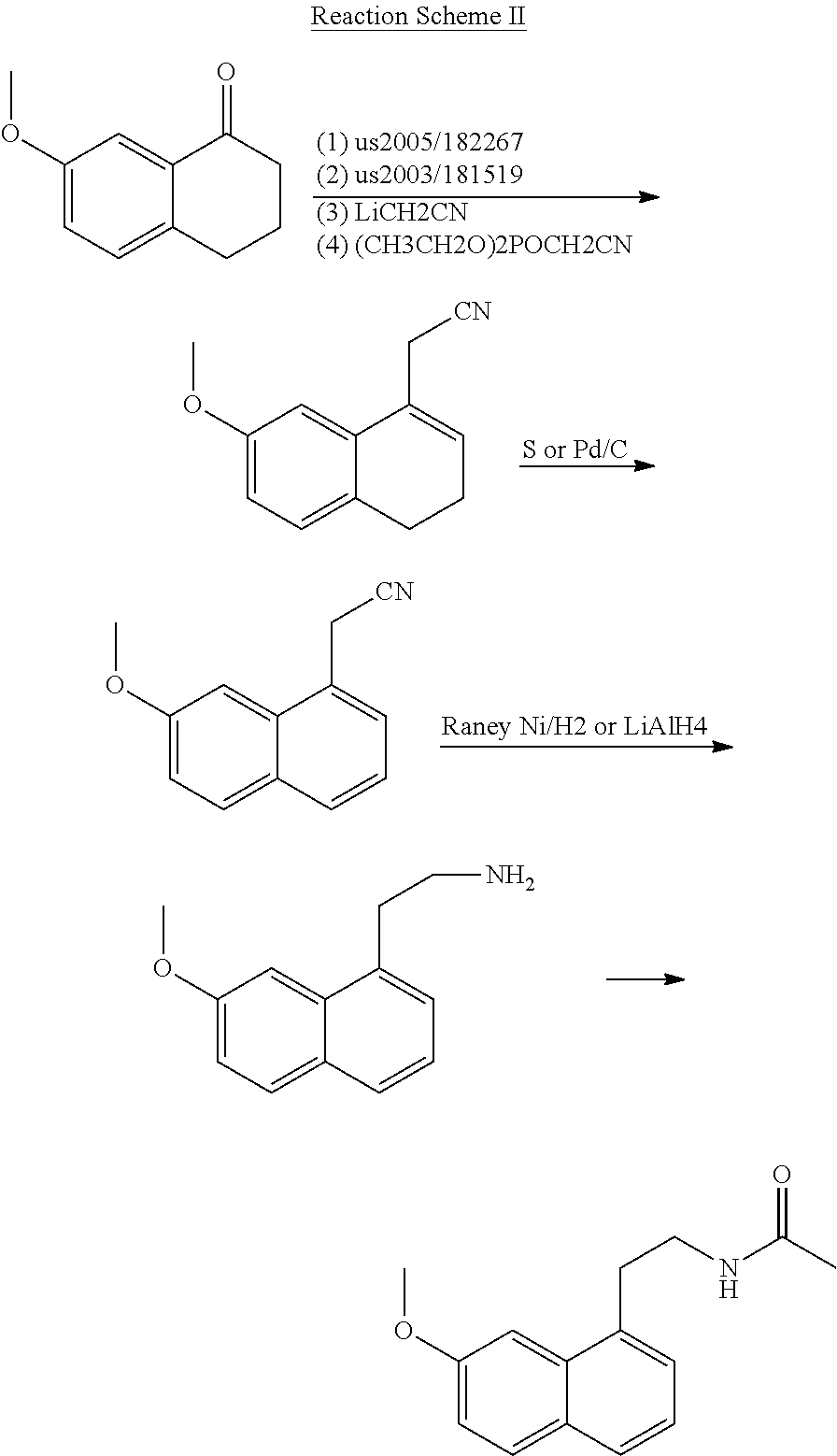
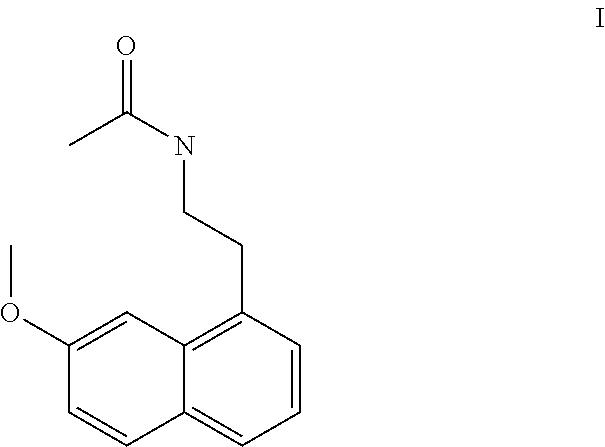
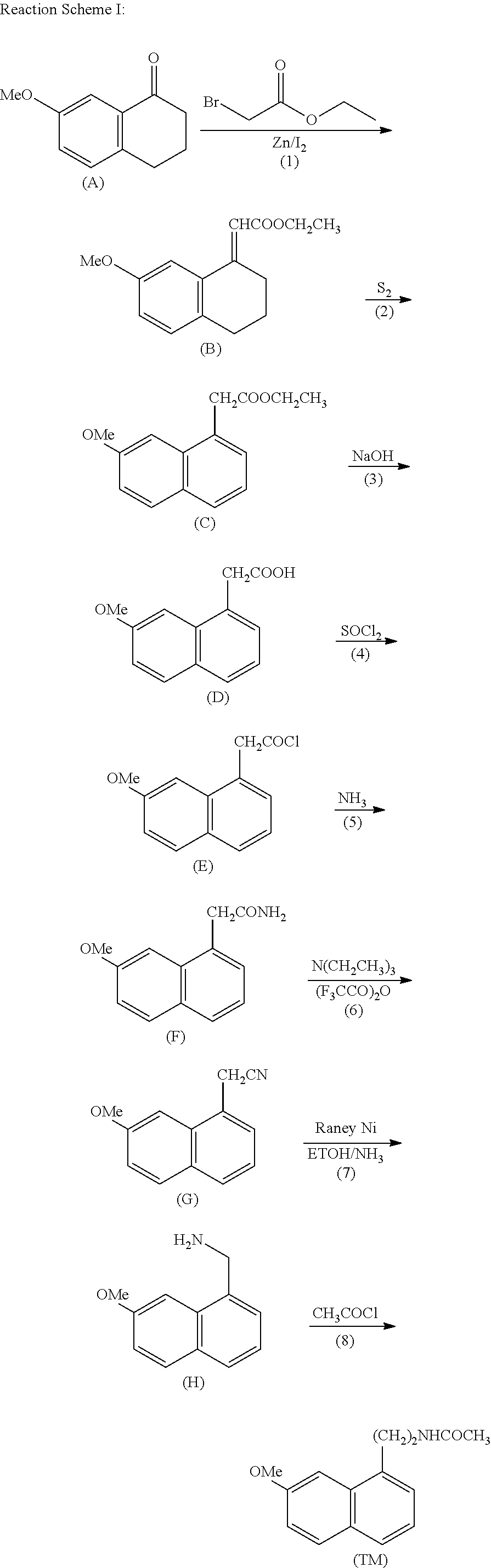
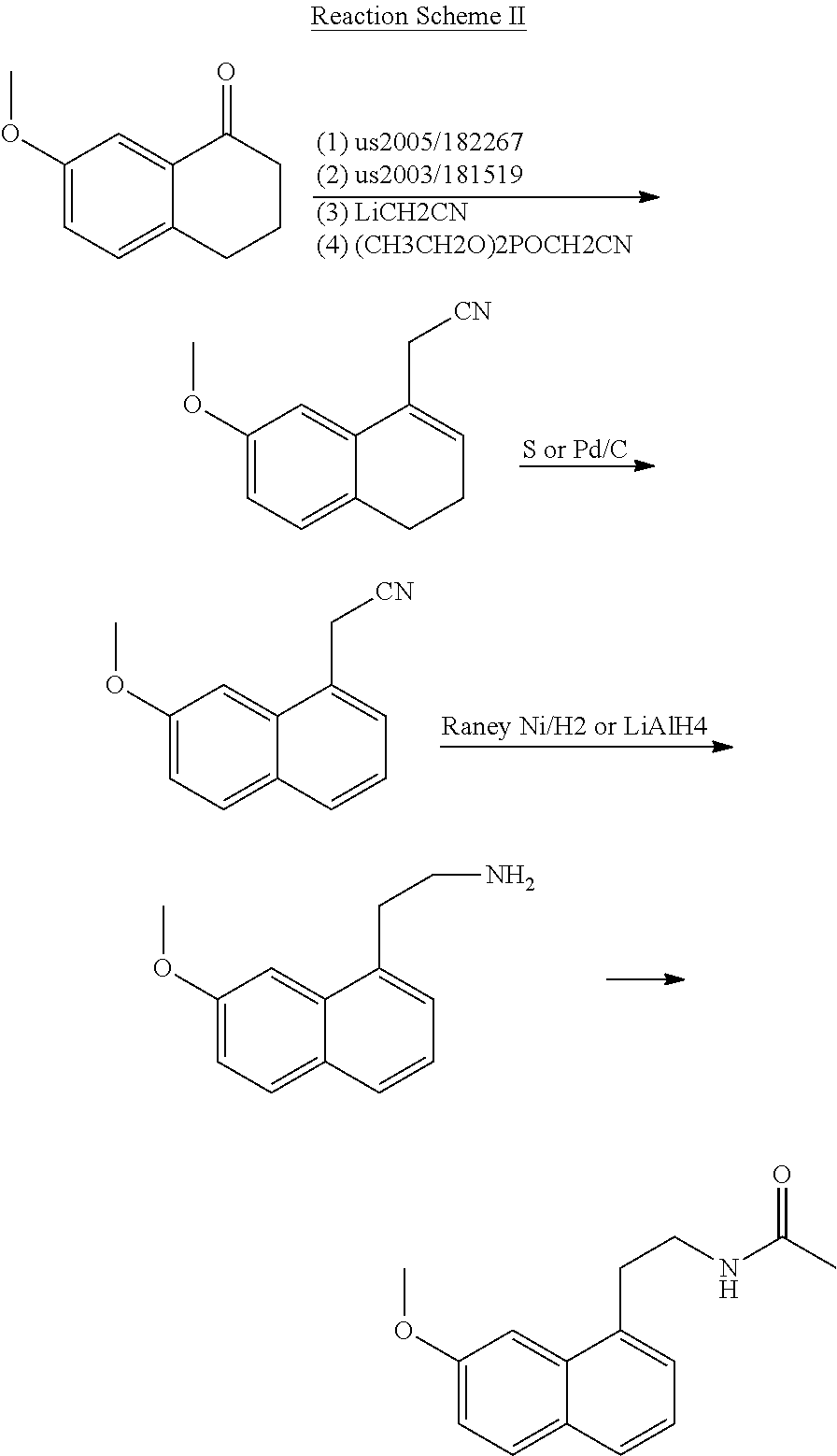
A process for the manufacture of agomelatine and its intermediate N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide is provided and includes reacting 7-methoxy-1-naphthyl ethanol (III) with benzenesulfonyl chloride to obtain 7-methoxy-1-naphthylethyl benzene sulfonate (IV), which is reacted with potassium phthalimide to produce N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide (II); and subjecting N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide (II) to alkaline hydrolysis and acetylation, to obtain agomelatine.
Owner:NHWA PHARMA CORPORATION
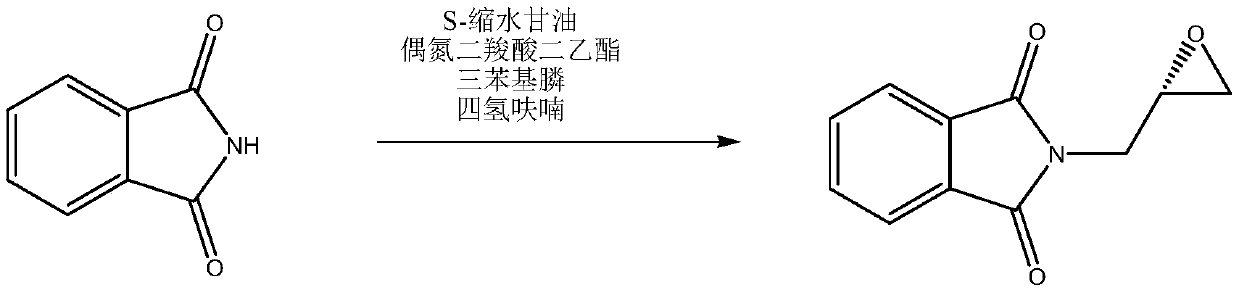
Preparation method of S-glycidylphthalimide
The invention relates to a preparation method of S-glycidylphthalimide. The preparation method is characterized in that in a reaction of potassium phthalimide or phthalimide and (S) epichlorohydrin, a phase transfer catalyst and potassium iodide are used so that S-glycidylphthalimide synthesis is realized. Compared with the prior art, the preparation method greatly improves an S-glycidylphthalimide yield, has simple and safe processes, produces high-purity products, has a low cost and is suitable for industrial production of S-glycidylphthalimide.
Owner:CHENGDU GUOHONG PHARMA
Synthesis method of 1,4-butanediamine
ActiveCN101735067ALow priceSimple process conditionsOrganic compound preparationAmino compound preparationSynthesis methodsPotassium
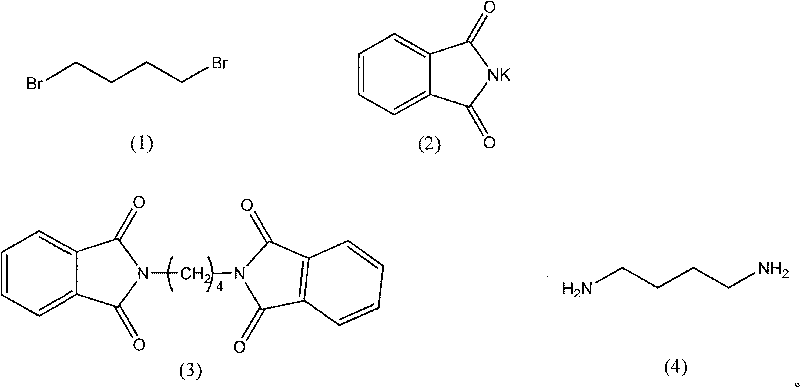
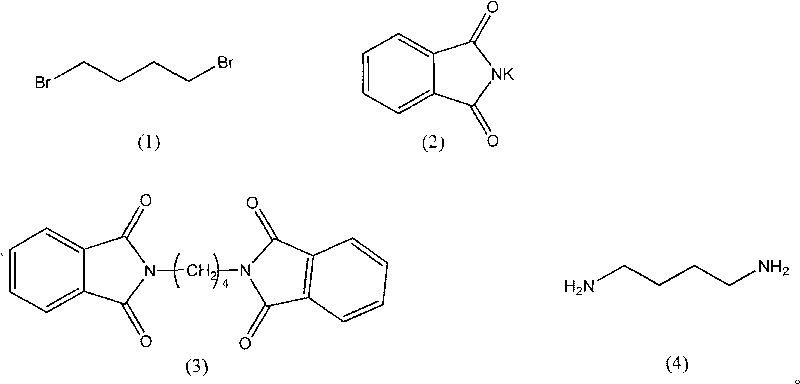
The invention provides a synthesis method of 1,4-butanediamine, which comprises the steps of: (a) dissolving the 1,4-dibromobutyl amine represented by formula (1) in a solvent A, adding the phthalimide potassium represented by formula (2), reacting while stirring at a reflux temperature, filtering reaction liquid, washing by using distilled water to obtain the 1,4-diphthalimide butane representedby formula (3); (b) adding the 1,4-diphthalimide butane obtained in the step (1) and 40% methylamine water solution to a solvent B, heating until solids are dissolved completely, stirring and reacting for 0.5-4 hours at the reflux temperature to obtain reaction products; and (c) filtering the reaction products, post-treating filtrate, distilling the filtrate under normal pressure, and collecting the fractions between 158 DEG C and 160 DEG C to obtain the 1,4-butanediamine. The invention has simple and convenient technological conditions, cheap raw materials, mild reaction conditions, easy control and convenient industrial production.
Owner:ZHEJIANG UNIV OF TECH
Cyclamine alkylamide ferulate compound as well as preparation method and application thereof
InactiveCN105777614AGood inhibitory effectGood selective inhibitionNervous disorderOrganic chemistryChemical structureChemical reaction
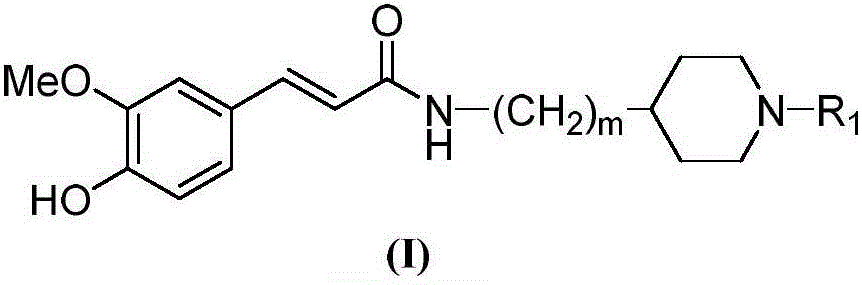
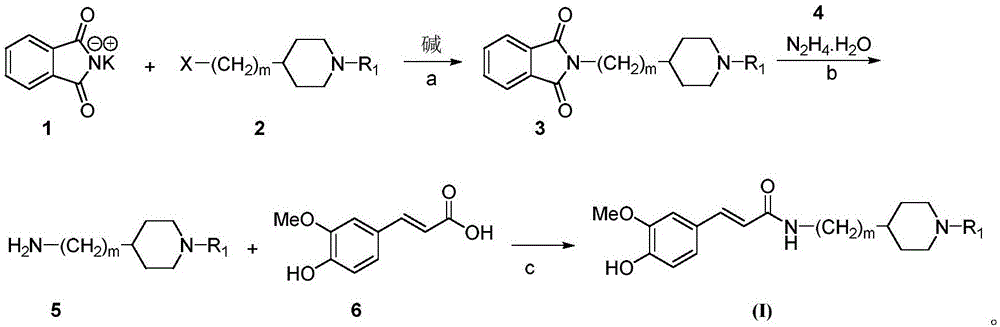
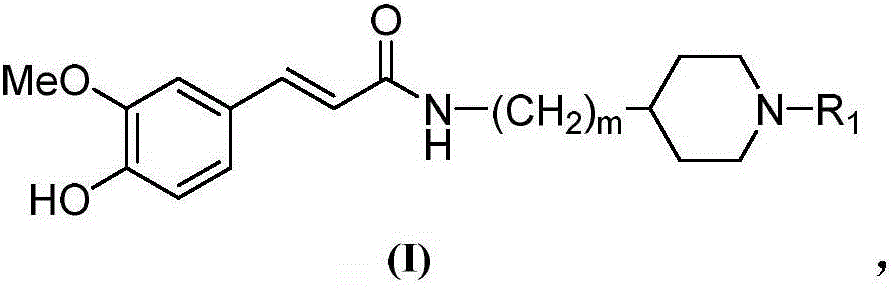
The invention belongs to the technical field of organic synthesis and particularly relates to a cyclamine alkylamide ferulate compound as well as a preparation method and application thereof. The preparation method comprises the following steps: enabling potassium phthalimide serving as a start raw material to react with 1-substituted-4-haloalkyl piperidine under the effects of a solvent and alkali to obtain a phthalimide alkylamine compound; performing hydrazinolysis of the phthalimide alkylamine compound and hydrazine hydrate to obtain a primary amine compound; and adding ferulic acid, a condensing agent and a solvent into the primary amine compound for a condensation reaction to obtain a product of cyclamine alkylamide ferulate compound. The cyclamine alkylamide ferulate compound provided by the invention is of a simple chemical structure, the chemical reactions in the preparation process are thorough, the product yield is high, the operation is convenient, the cost is low, and the product can be used for perfectly treating the neurodegenerative diseases.
Owner:NANYANG NORMAL UNIV
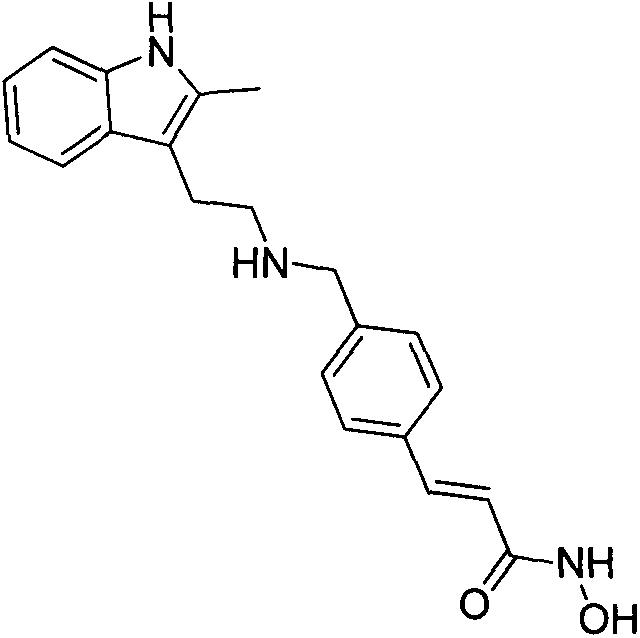
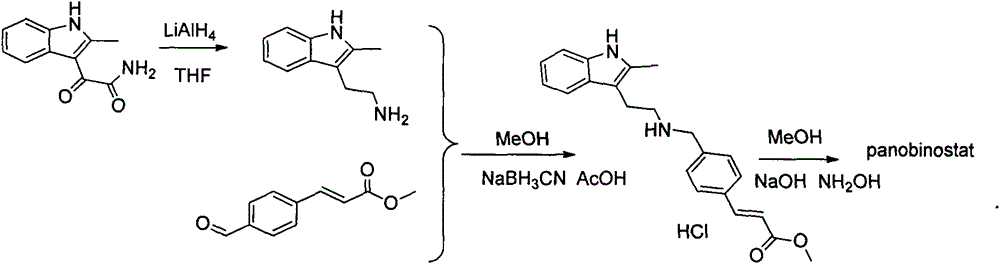
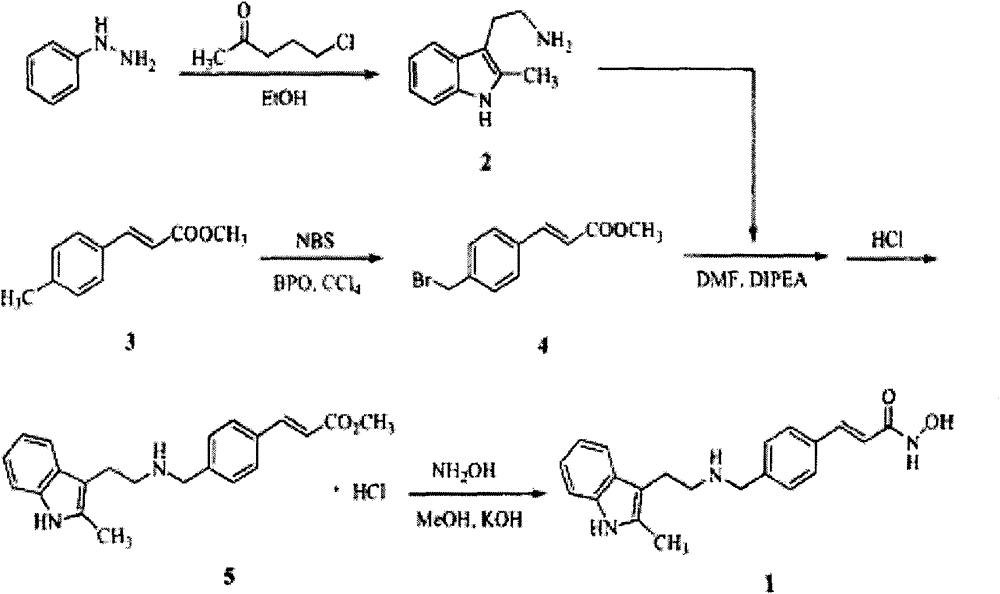
Synthesis method of panobinostat
InactiveCN106674079ASecurity impactSuitable for industrial productionOrganic chemistryTryptaminePanobinostat
The invention discloses a synthesis method of panobinostat. The synthesis method comprises a step of synthesizing 2-methyl tryptamine or hydrochloride thereof, namely taking 2-methylindole as a raw material, conducting reaction with chloroacetyl chloride or bromoacetyl bromide, and conducting reaction with potassium phthalimide to obtain the 2-methyl tryptamine. According to the technical scheme adopted by the invention, influence of toxic raw materials on the safety of a panobinostat product is avoided; meanwhile, the invention provides the synthesis method applicable to industrial production.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Method for preparing nitrogen-doped carbon material with high specific surface area from phthalimide potassium
ActiveCN110734049ASimple manufacturing processLarge specific surface areaProductsReagentsImidePorous carbon
The invention discloses a method for preparing a high-specific-surface-area nitrogen-doped carbon material from phthalimide potassium. The method comprises the following steps: carbonizing phthalimidepotassium at a high temperature, adding hydrochloric acid into an obtained carbonized product for treatment, washing a product obtained after pickling repeatedly with deionized water, and drying to obtain the nitrogen-doped porous carbon material. In-situ activation effect is achieved by using potassium element contained by the carbon material, no activating agent is needed, a developed porous structure is formed through one-step reaction, and the specific surface area of the carbon material can reach 2000 m<2> / g or above; meanwhile, nitrogen doping of the carbon material is realized by utilizing nitrogen element contained in phthalimide potassium; the prepared nitrogen-doped carbon material has a high specific surface area and high porosity, and shows a good adsorption characteristic oncarbon dioxide.
Owner:INST OF APPLIED CHEM JIANGXI ACAD OF SCI
A kind of preparation method of S-glycidyl phthalimide
The invention relates to a reaction of potassium phthalimide or phthalimide and (S) epichlorohydrin, adding a phase transfer catalyst and potassium iodide to synthesize S-glycidyl phthalate A new approach to formimides. Compared with the prior art, the synthesis method can greatly improve the yield of the obtained S-glycidyl phthalimide product, and the operation is simple, the technological process is simple and safe, the product has high purity and low cost, and is extremely suitable for industrial production of S-glycidyl phthalimide. - Glycidylphthalimide.
Owner:CHENGDU GUOHONG PHARMA
Active organo montmorillonite and preparation method thereof
InactiveCN101531373AIncrease layer spacingGood dispersionSilicon compoundsDispersityHydrazine compound
The invention discloses an active organo montmorillonite and a preparation method thereof, wherein, the organo montmorillonite is the amino-containing active organo montmorillonite composed of Na-montmorillonite and an organic intercalation agent which intercalate in the Na-montmorillonite and is subjected to the modification of potassium phthalimide; the organic intercalation agent is an organic compound represented by R(CH2)<12-18N>(CH3)3R or R(CH2)<12-18>NH2 structural formulae, R in the formulae is chlorine atom or bromine atom. The preparation method thereof comprises the step of: subjecting the modified organic intercalation agent and the Na-montmorillonite to intercalation in a solvent at 80-120 DEG C and then to amination with hydrazine hydrate on condition that the solvent is present, in order to prepare the amino-containing active organo montmorillonite. The organo montmorillonite prepared by the invention has outstanding dispersity both in the organic solvent and in the water and can be better used for blending with other polymers to prepare a composite material. The invention can be used for the fields of plastic processing, the preparation of chemical building material, the preparation of nano material, biomedical material and cosmetics.
Owner:SICHUAN UNIV
Thioether allyl isothiocyanate compounds, and preparation method and applications thereof
InactiveCN105237451AHigh anticancer activityLow genotoxicityOrganic chemistryAntineoplastic agentsAlkaneBenzoyl bromide
The invention provides a series of novel thioether allyl isothiocyanate compounds with a general structure disclosed in the invention, and a simple method used for rapid synthesis of the thioether allyl isothiocyanate compounds. According to the method, double-terminal halogen-substituted alkanes are taken as initial raw materials, are reacted with phthalimide potassium so as to introduce N atoms, are reacted with sodium hydrosulfide so as to produce mercaptan, and mercaptan is reacted with benzyl bromide or is reacted with a heterocyclic compound with sulfydryl so as to introduce the thioether structure, an obtained product is reacted with hydrazine hydrate so as to obtain a primary amine, and the primary amine is reacted with an alkali, carbon disulfide, and methylsufonyl chloride so as to prepare the thioether allyl isothiocyanate compounds with heterocyclic nitrogen structures. The method contains few steps; operation is simple; the obtained products can be easily purified; and yield is high. The series of novel thioether allyl isothiocyanate compounds possess obvious killing activity on tumor cells, and possess obvious killing activity on cervical carcinoma cells and lung cancer cells.
Owner:HAINAN NORMAL UNIV
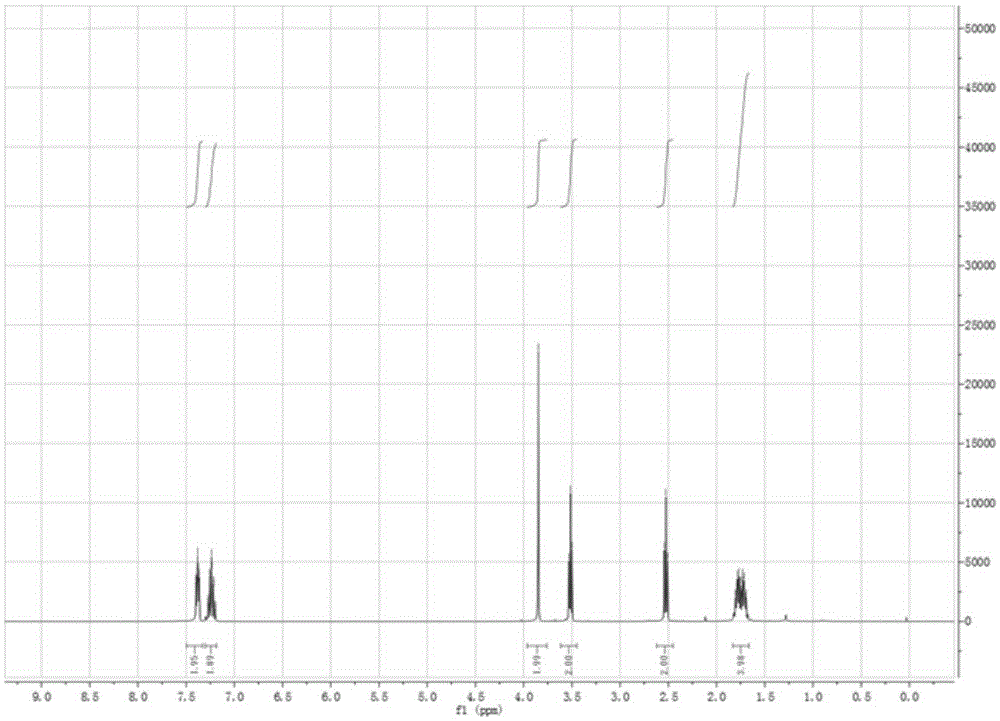
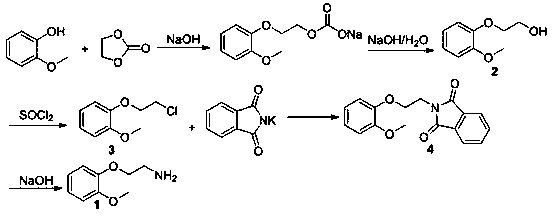
Preparation method for 2-(2-methoxyphenoxy)ethylamine
InactiveCN109206328AReduce manufacturing costPromote safe productionOrganic compound preparationAmino-hyroxy compound preparationGuaiacolPotassium phthalimide
The invention provides a preparation method for 2-(2-methoxyphenoxy)ethylamine. The preparation method comprises the following steps: synthesizing 2-(2-methoxyphenoxy)ethanol with guaiacol as a starting material; then synthesizing 2-(2-methoxyphenoxy)chloroethane through chlorination; then reacting 2-(2-methoxyphenoxy)chloroethane with potassium phthalimide to obtain N-(o-methoxyphenoxyethyl)-phthalimide; and finally, performing basic hydrolysis to obtain 2-(2-methoxyphenoxy)ethylamine. The yields of the above four steps of reactions are that the yield of 2-(2-methoxyphenoxy)ethanol is 98.9%;the yield of 2-(2-methoxyphenoxy)chloroethane is 93.7%; the yield of N-(o-methoxyphenoxyethyl)-phthalimide is 86.4%; the yield of 2-(2-methoxyphenoxy)ethylamine is 91.2%; and the total yield of the four steps is 73.04%, which is higher than the yield of 43% in conventional production processes. The preparation method of the invention reduces the production cost of 2-(2-methoxyphenoxy)ethylamine and is safe in the production process.
Owner:TONGREN UNIV
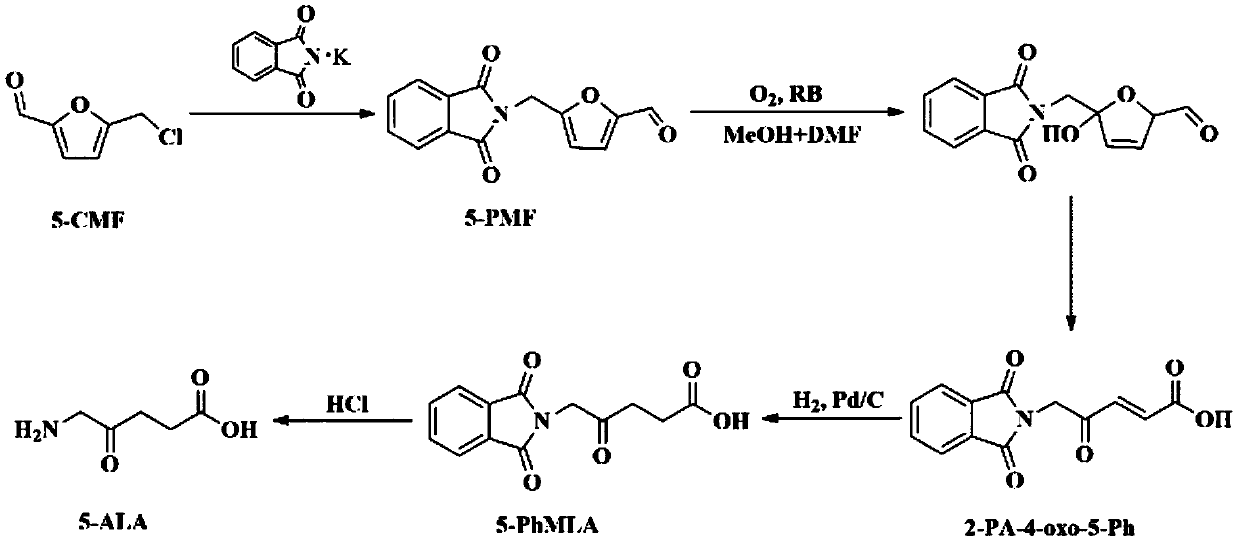
Process for preparing 5-aminolevulinic acid from 5-chloromethyl furfural
InactiveCN110330440AAvoid high cost, high risk and other problemsAvoid high risk, harsh experimental operating conditions and other problemsOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisAmino-Levulinic Acid
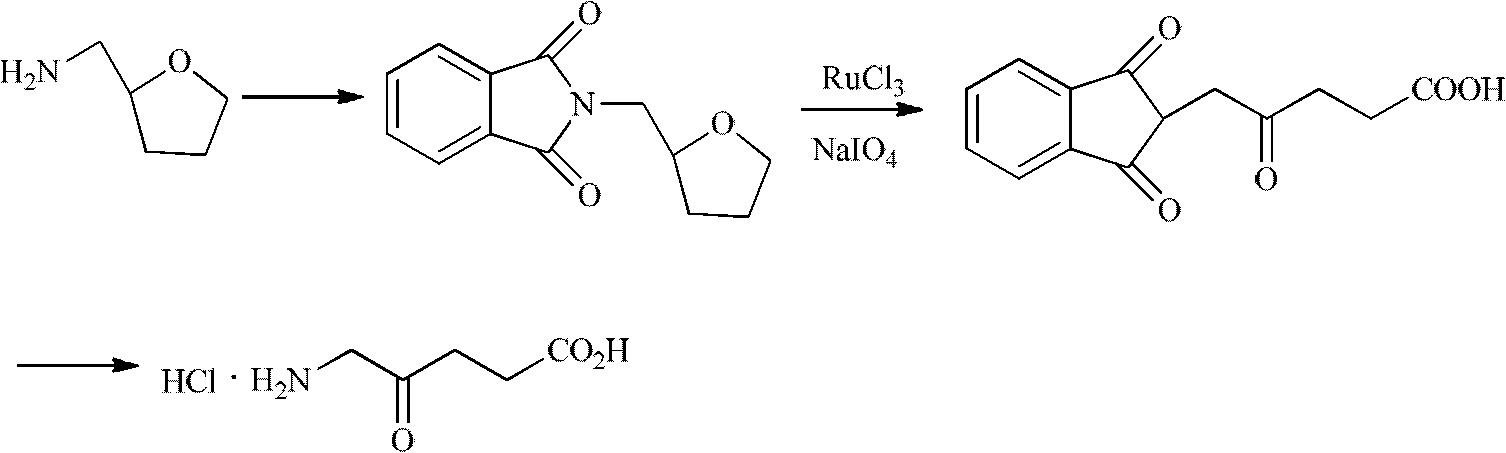
The invention discloses a process for preparing 5-aminolevulinic acid from 5-chloromethylfurfural. The biomass-based 5-chloromethylfurfural and cheap and easily available potassium phthalimide are used as reaction raw materials to react to obtain ammoniated product 5-(phthalimide) methylfurfural with a yield of 97.1%, and then the 5-ALA is obtained through synthesis steps of photooxidation, reduction, hydrolysis and the like. The structure of the 5-ALA is determined by nuclear magnetic resonance. The purity of the 5-ALA is 96.1% and the total reaction yield is 23.7%. Compared with the traditional chemical synthesis route of the 5-aminolevulinic acid, the process has the advantages of cheap and easily available raw materials, simple and convenient operation, mild reaction conditions, no toxicity and the like, and has a good industrial popularization prospect.
Owner:XIAMEN UNIV
Method for synthesizing diamine terminated polystyrene based on ATRP active polymerization method and application of diamine terminated polystyrene
The invention provides a method for synthesizing diamine terminated polystyrene based on an ATRP active polymerization method and an application of the diamine terminated polystyrene. The method comprises the steps as follows: reacting potassium phthalimide and dibromomethane in anhydrous DMF, and carrying out purification to obtain an initiator N-bromomethyl phthalimide; then, carrying out atom transfer radical polymerization on the initiator and a styrene monomer under the action of a catalyst, and carrying out a reaction at 110 DEG C for 0.5-3 h to generate modified polystyrene with different molecular weights; next, reacting the modified polystyrene and potassium phthalimide in the anhydrous DMF to obtain polystyrene protected by a bisphthalimide group; and finally, reducing the polystyrene protected by a bisphthalimide group by hydrazine hydrate to obtain diamine terminated polystyrene. Then, the synthesized diamine terminated polystyrene is compounded with functional nanoparticles, so that the oil / water interfacial tension can be greatly reduced by 85% or above, and the synthesized diamine terminated polystyrene can be applied to the field of emulsion demulsification and thelike.
Owner:SOUTHWEST PETROLEUM UNIV
Process for the manufacture of agomelatine and its intermediate
ActiveUS8653281B2Easy to implementHigh purityNervous disorderOrganic compound preparationAcetylationPotassium phthalimide
A process for the manufacture of agomelatine and its intermediate N-[2-(7-methoxy- 1-naphthy)ethyl]phthalimide is provided and inclues reacting 7-methoxy-1-naphthyl ethanol (III) with benzenesulfonyl chloride to obtain 7-methoxy-1 -naphthylethyl benzene sulfonate (IV), which is reacted with potassium phthalimide to produce N-[2-(7-methoxy-1-naphthy)ethyl]phthalimide (II); and subjecting N-[2-(7-methoxy-1-naphthy)ethyl]phthalimide (II) to alkaline hydrolysis and acetylation, to obtain agomelatine.
Owner:NHWA PHARMA CORPORATION
Preparation method of silodosin key intermediate
PendingCN114751852ASplit atom utilization is lowReduce utilizationOrganic compound preparationCarboxylic acid salt preparationTrichloroacetonitrilePyran
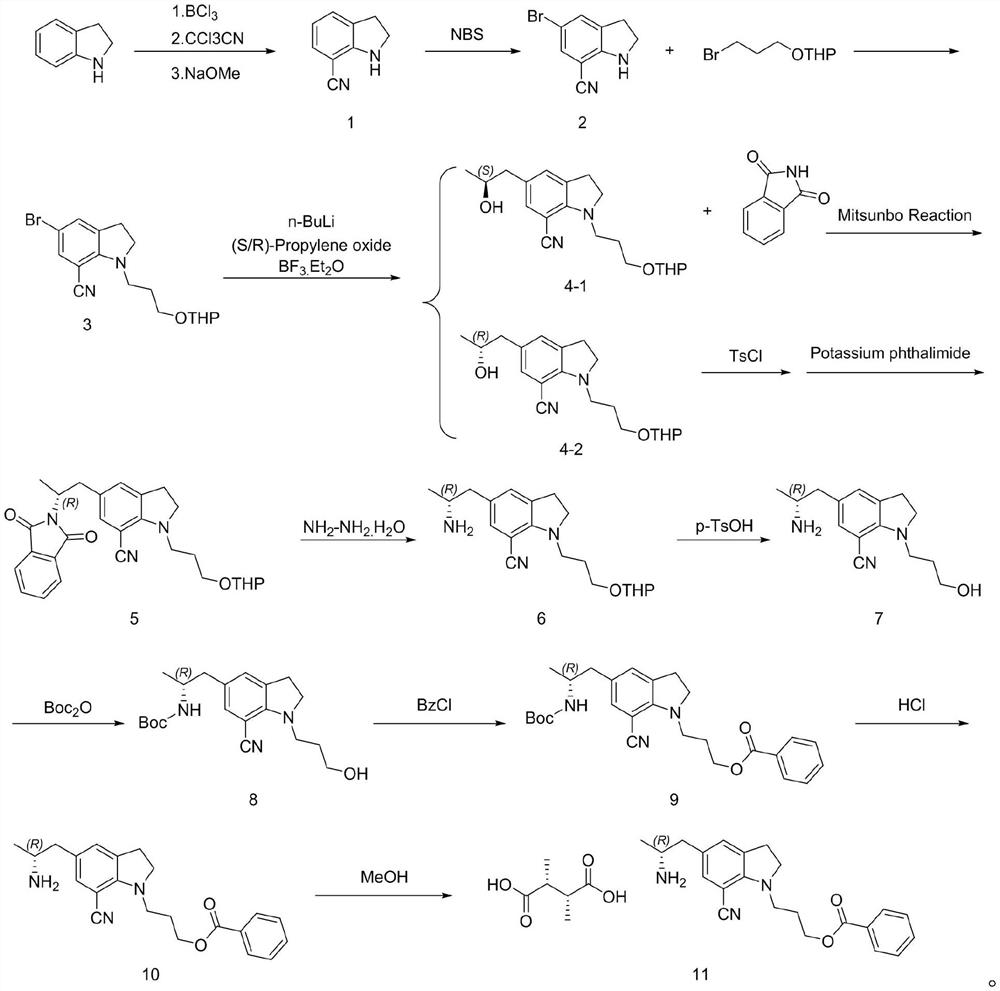
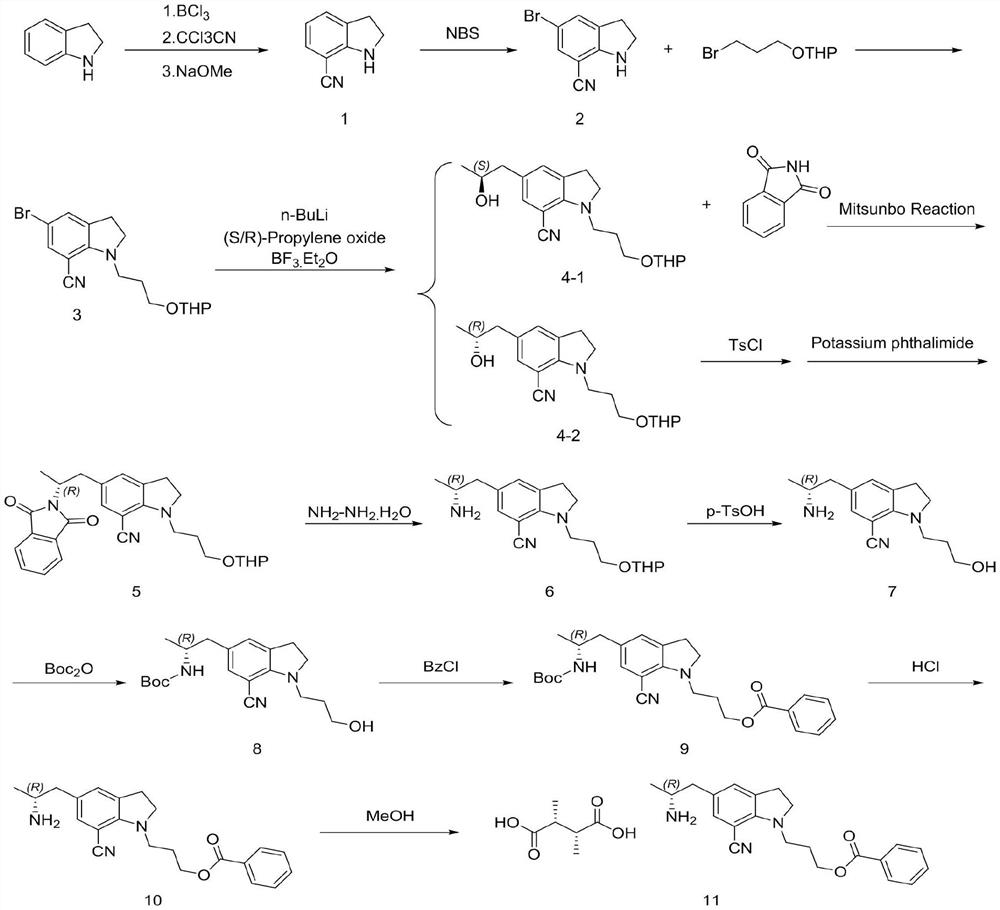
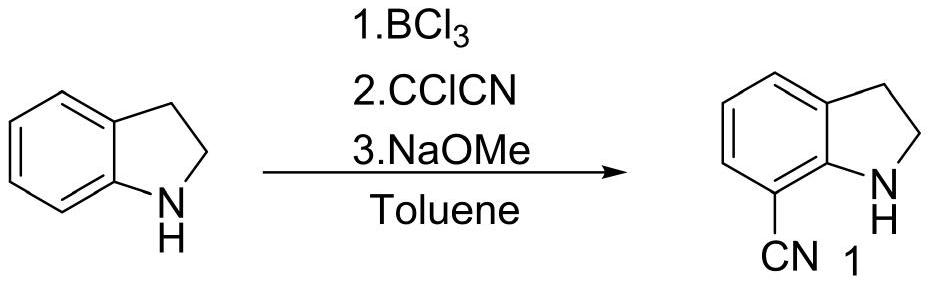
The invention discloses a preparation method of a silodosin key intermediate, and belongs to the technical field of medicine synthesis. Carrying out Friedel-Crafts reaction on indoline and trichloroacetonitrile to obtain a compound 1; brominating to obtain a compound 2; then carrying out substitution with 2-(3-bromopropoxy) tetrahydro-2H-pyran to obtain a compound 3; then carrying out nucleophilic addition with (S)-epoxypropane or (R)-epoxypropane at an ultralow temperature to obtain a compound 4; then carrying out Mitsunobu reaction with phthalimide to obtain a compound 5 (configuration inversion) or esterifying with paratoluensulfonyl chloride, and reacting with potassium phthalimide under the condition of inorganic alkali to obtain the compound 5; reducing through hydrazine hydrate to obtain a compound 6; then removing tetrahydropyran protection from p-toluenesulfonic acid to obtain a compound 7; performing amino Boc protection under an alkaline condition to obtain a compound 8; esterifying with benzoyl chloride to obtain a compound 9; removing Boc protection with hydrochloric acid to obtain a compound 10; and salifying with L-tartaric acid to obtain a compound 11. Compared with the prior art, the preparation method has the advantages that cyano groups synthesized by Vilsmeier reaction, hydroxylamine oximation and acetic anhydride dehydration at the 7 site and introduction of amino groups at the 5 site through nitro groups or reductive amination are avoided, and heavy metals such as Pd / Pt / Zn and the like do not need to be used; the continuous operation of the whole steps is increased, the production cost of the silodosin intermediate is greatly reduced, and industrial large-scale production is facilitated.
Owner:山西库邦生物医药科技有限公司
Preparation method of functional amino proline
ActiveCN101830842ALow costRaw materials are easy to getOrganic chemistryPotassiumPotassium hydroxide
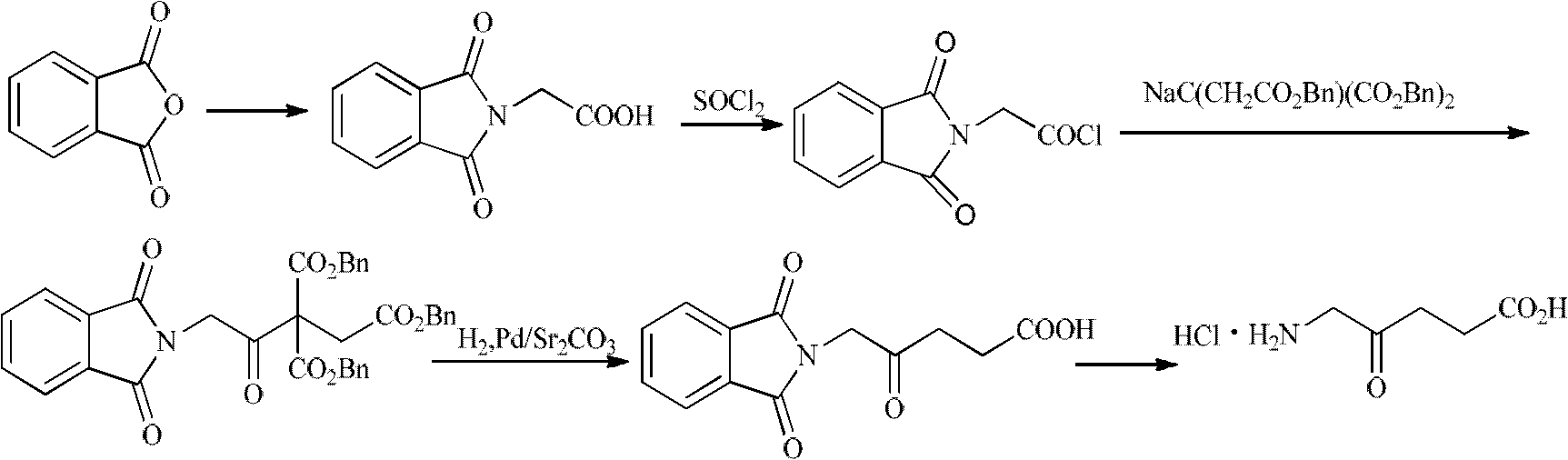
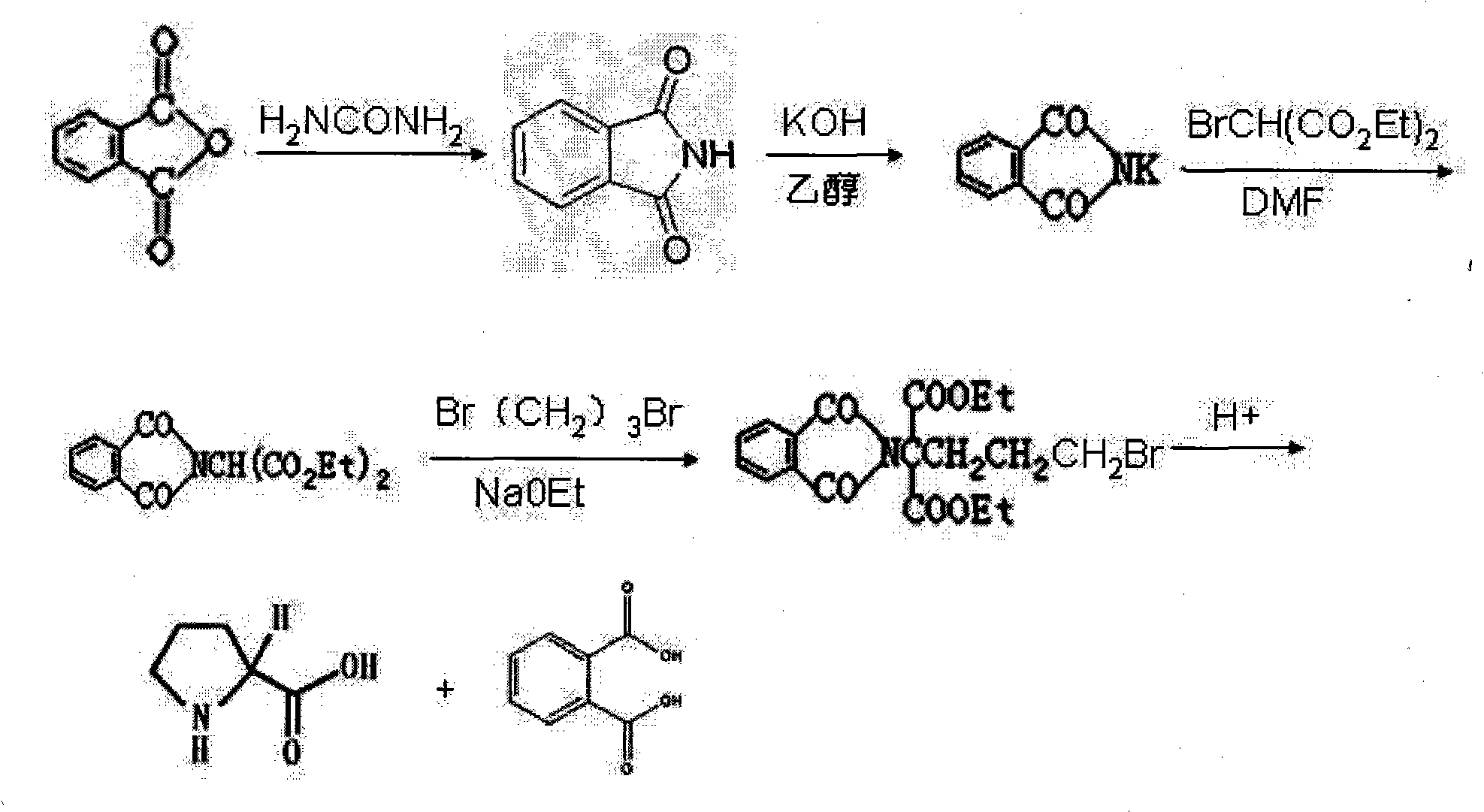
The invention discloses a preparation method of functional amino proline. The invention adopts the technical scheme that phthalic anhydride and urea are reacted to obtain phthalimide, the obtained phthalimide is dissolved into ethanol, the mixture is regulated to alkalinity by potassium hydroxide to generate phthalimide potassium, the phthalimide potassium is reacted with bromodiethyl malonate to obtain phthalyl diethyl malonate, the phthalyl diethyl malonate is reacted with 1,3-dibromopropane to generate phthalyl-2-bromopropyl diethyl malonate, the phthalyl-2-bromopropyl diethyl malonate is dissolved into the ethanol to be regulated to the alkalinity, and then the mixture is reacted with acid to generate a product of proline. Compared with the traditional preparation method of the proline, the invention can be repeatedly utilized by adopting phthalic anhydride as raw materials, lower the cost and also save expensive sodium borohydride reducing agent needed by the traditional prepared proline, therefore, the invention has the advantages of low cost, simple process, high yield and high purity and is more beneficial to mass production.
Owner:卫仕宠物营养研究院(芜湖)有限公司
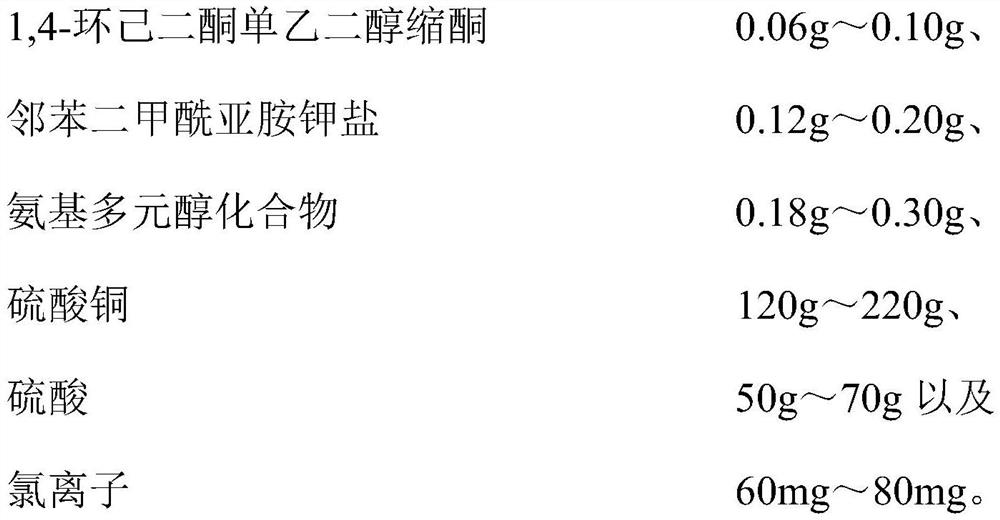
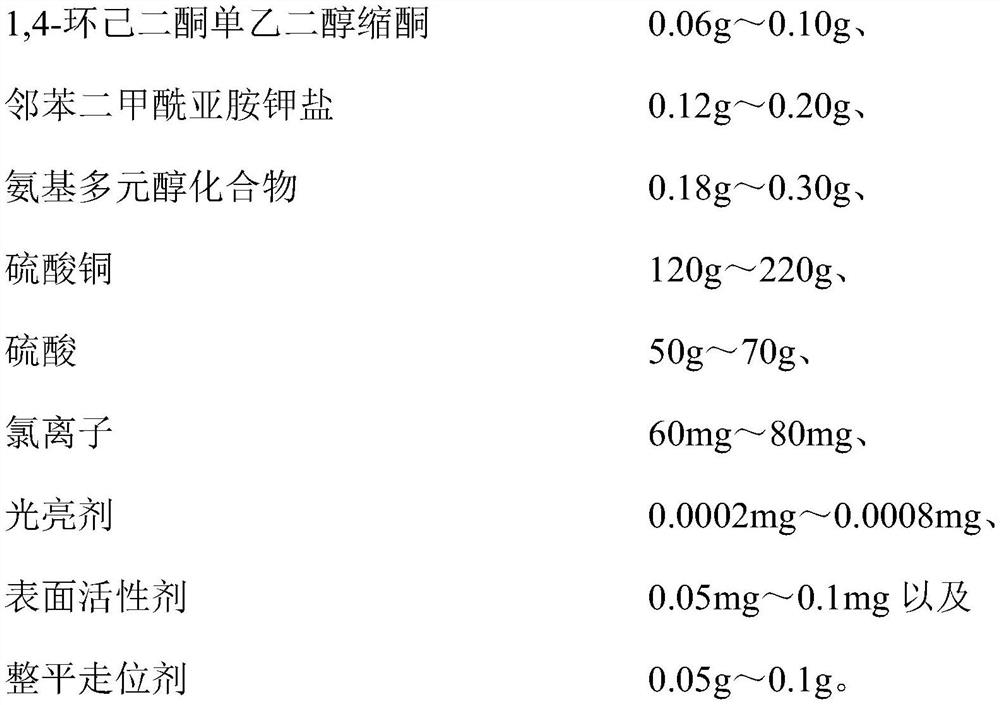
Copper plating additive, copper plating solution and application thereof
The invention relates to a copper plating additive, a copper plating solution and application thereof. The copper plating additive comprises 1, 4-cyclohexanedione monoethylene ketal, a potassium phthalimide salt and an amino polyol compound. Wherein the 1, 4-cyclohexanedione monoethylene glycol ketal can obviously improve the brightness of a brush plating copper layer, the phthalimide potassium salt can effectively improve the dissolving efficiency of the 1, 4-cyclohexanedione monoethylene glycol ketal, meanwhile, the effects of refining grains, leveling and improving the uniformity and thickness of a plating layer are achieved, and the amino polyalcohol compound can greatly change the color tone of the copper layer; the color is brighter. In addition, the copper plating additive has the advantages of being environmentally friendly, non-toxic, low in cost and the like, and is suitable for large-scale industrial production. When the copper plating additive is added into a copper plating solution, the thickness, the brightness and the plumpness of a plating layer can be greatly improved by adding a very small amount of the copper plating additive, and the stability of the original copper plating solution and the performance of the original copper plating layer are not changed.
Owner:JIANGYIN NANOPORE INNOVATIVE MATERIALS TECH LTD
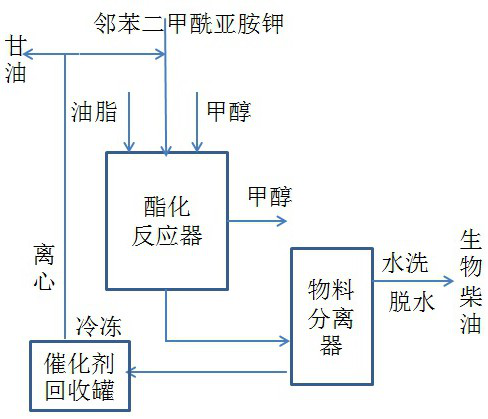
Biodiesel synthesized by catalyzing vegetable fat with potassium phthalimide
PendingCN114717055AEasy to operateIncrease conversion rateFatty acid esterificationOrganic-compounds/hydrides/coordination-complexes catalystsImideVegetable oil
The invention discloses a method for synthesizing biodiesel by using potassium phthalimide to catalyze a reaction between vegetable fat and methanol. And the solid potassium phthalimide catalyst can be recycled. The process has the characteristics of low transesterification reaction temperature, short time, simple production process, light glycerol color, few byproducts, no three-waste pollution and the like. In addition, the potassium phthalimide is dissolved in methanol, so that homogeneous reaction during transesterification is realized. Along with the esterification process, the catalyst is dissolved into a glycerol phase and is easily separated from a reaction system by virtue of density difference. And placing the glycerol phase material in an environment of 0-5 DEG C for 10 hours, so that the potassium phthalimide can be separated out from the glycerol phase. After centrifugal separation, regeneration of the solid catalyst can be completed by washing with a small amount of ethanol, so that heterogeneous separation of reaction materials and catalyst recovery are realized.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of 4-aminobutanol
ActiveCN108658792ALow costHigh yieldOrganic compound preparationAmino-hyroxy compound preparationEconomic benefitsPotassium phthalimide
The invention provides a preparation method of 4-aminobutanol. The method comprises the steps of taking potassium phthalimide and 4-chlorbutanol as raw materials, and preparing an intermediate in thepresence of a solvent and a phase transfer catalyst; and then hydrolyzing the intermediate under the effect of an alkaline solution to obtain the 4-aminobutanol. The raw materials used in the method are simple, the cost is low, the reaction conditions are gentle, potential safety hazards do not exist, and additional reactions hardly exist in a reaction process of the various used raw materials; and in a preparation process, substances which are difficult to separate are not generated, and aftertreatment is simple and is easy to operate; and therefore, the yield of the 4-aminobutanol is greatlyimproved, the utilization rate of the raw materials is increased, and the production cost is reduced. Under the condition of low cost, the yield of products is increased obviously, and the economic benefit is remarkable.
Owner:HENAN CHEM IND RES INST
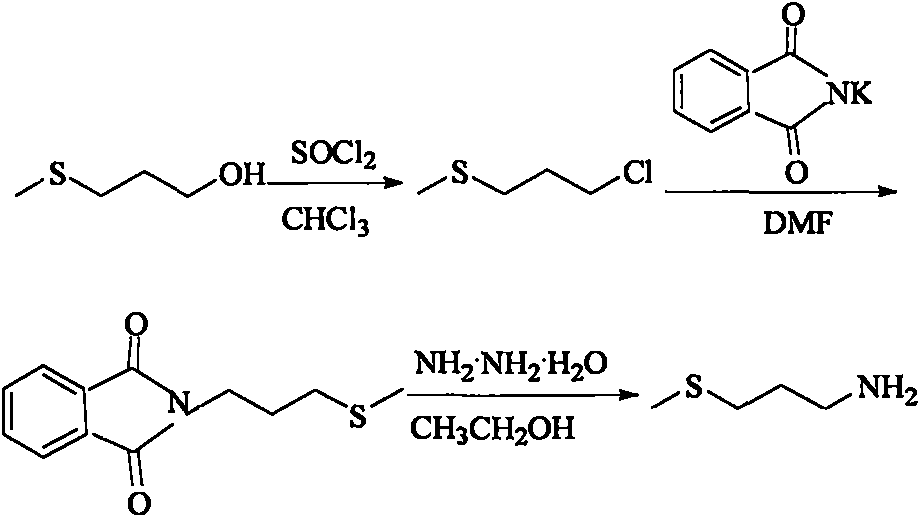
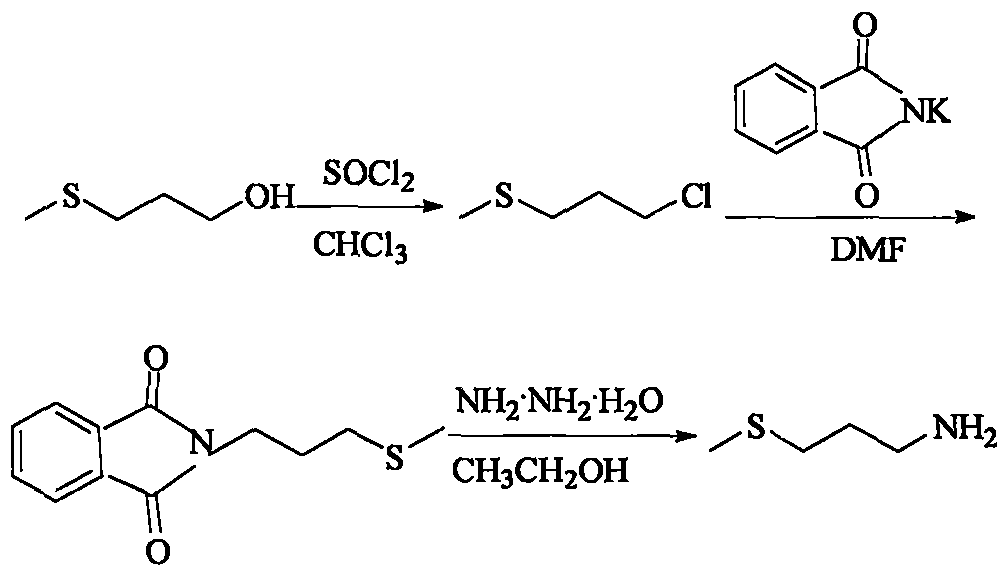
Method for preparing 3-methylthio propylamine
The invention provides a preparation method of 3-methylthio propylamine. The method is characterized in that under the effects of microwaves and ultrasonic waves, flavoring essence of 3-methylthio propyl alcohol capable of being easily obtained in China is used as a starting raw material; the reaction time is short; the total yield is relatively high. Under the effects of microwaves and ultrasonicwaves, firstly, 3-methylthio propyl alcohol and thionyl chloride react for 20 to 60 min under the back flow condition to prepare 3-methylthio-1-chloropropane, and the yield is greater than 95 percent; then, at 80 to 90 DEG C, the 3-methylthio-1-chloropropane and phthalimide potassium react for 0.5 to 2.5h to prepare N-3-methylthio propyl phthalimide, and the yield is greater than 94 percent; finally, the N-3-methylthio propyl phthalimide and hydrazine hydrate perform backflow reaction in absolute ethyl alcohol for 20 to 60min to prepare the 3-methylthio propylamine, and the yield is greater than 85 percent.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
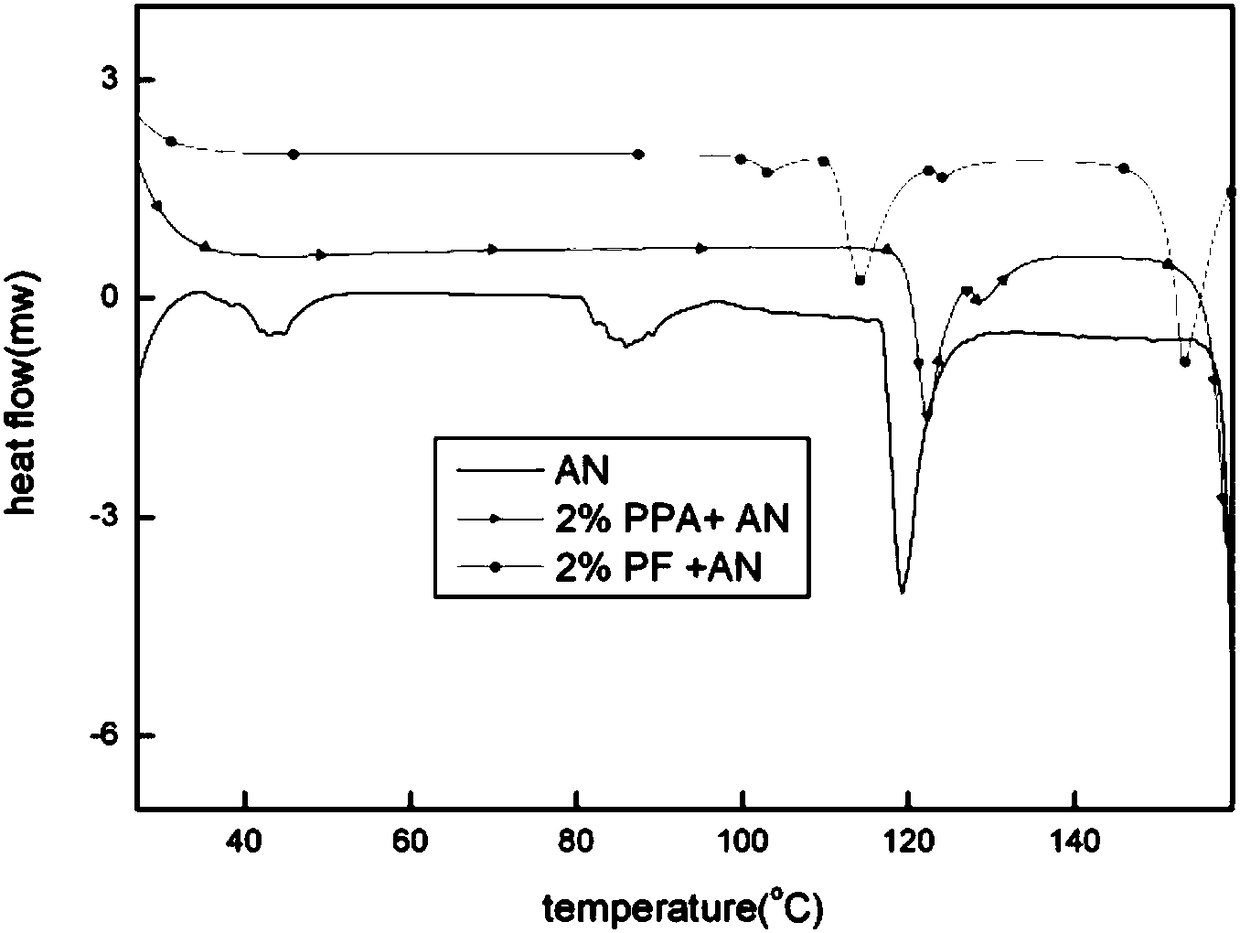
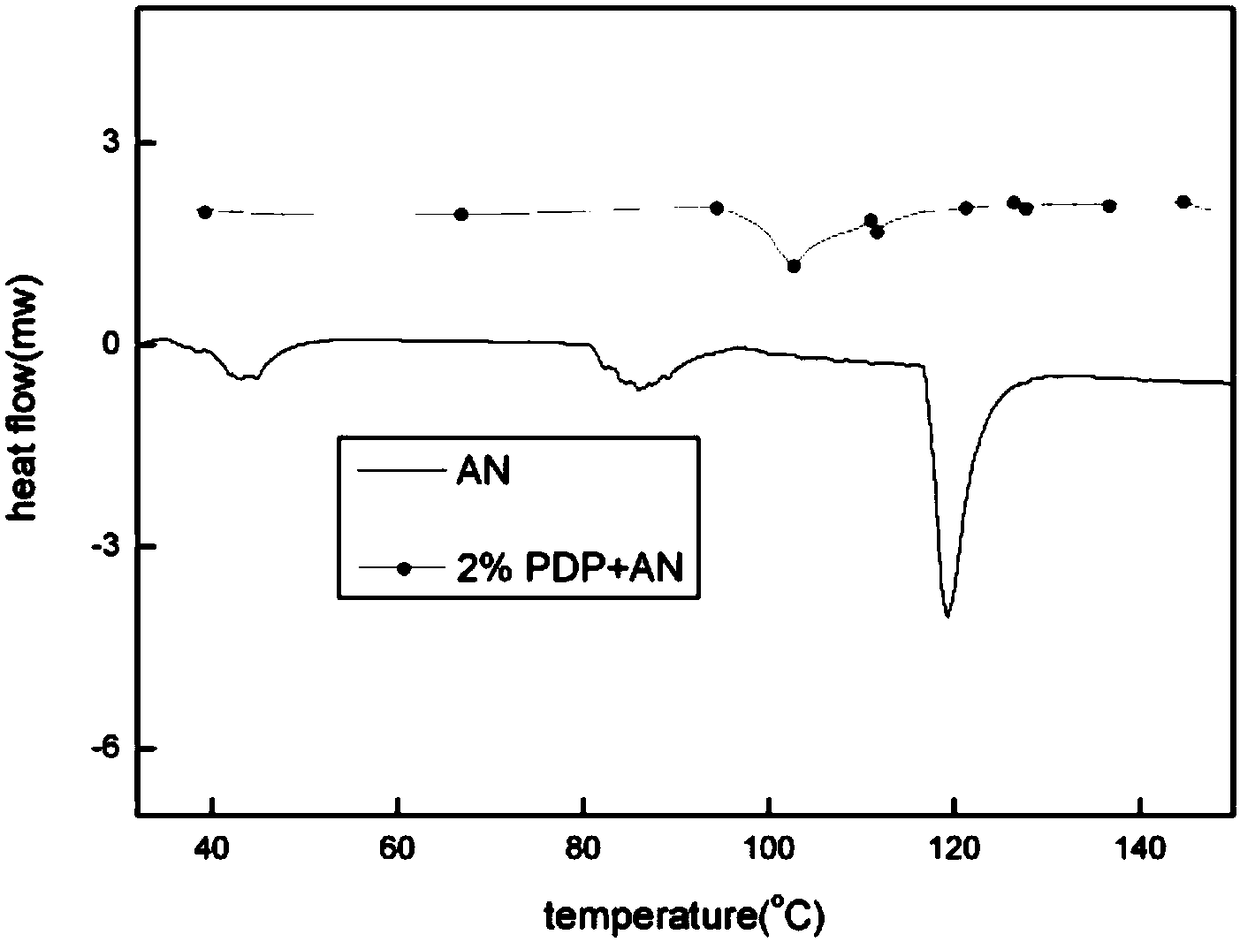
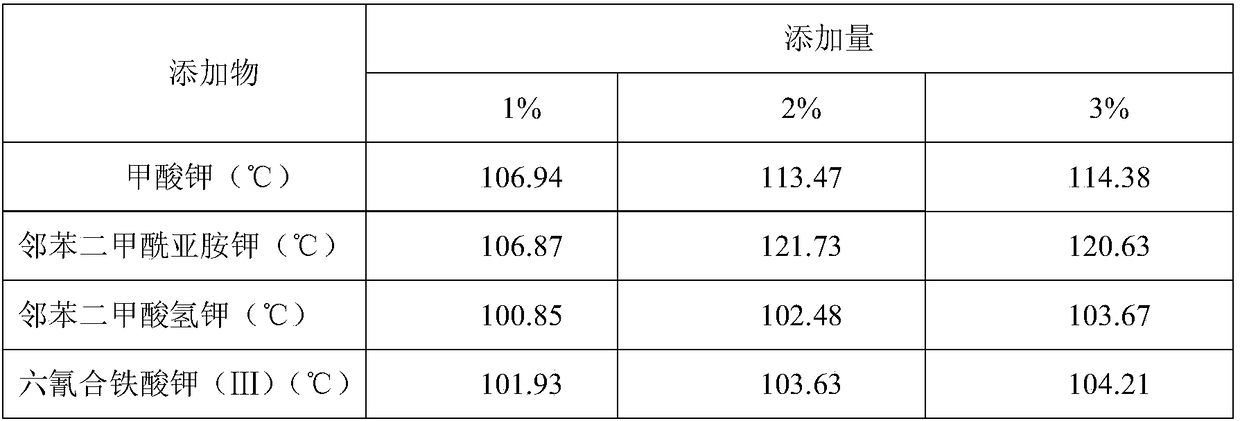
Preparing method for phase-stable ammonium nitrate
ActiveCN105801323AInhibition of phase transitionDoes not affect energyNon-explosive stabilisersOrganic acidPotassium ferricyanide
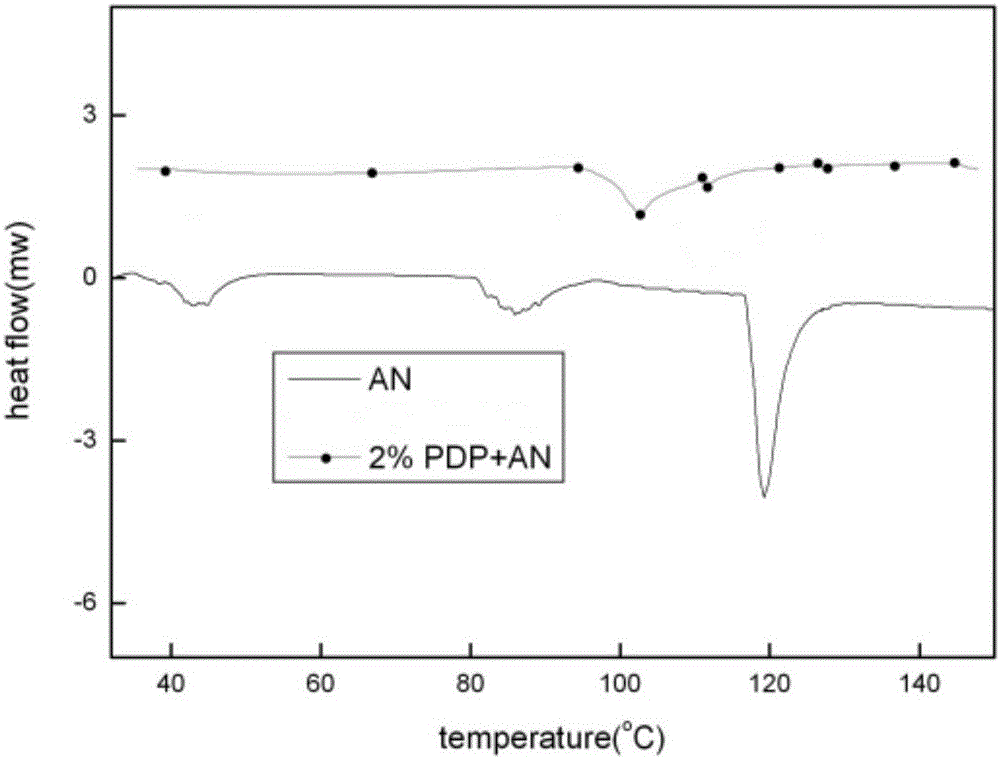
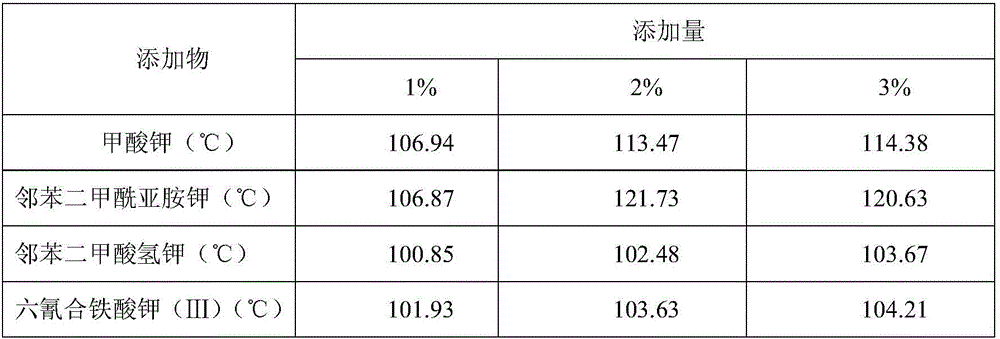
The invention discloses a preparing method for phase-stable ammonium nitrate.The preparing method comprises the following steps that dried, ground and smashed ammonium nitrate is dissolved in a solvent; a phase stabilizing additive with the adding amount 1-3% of ammonium nitrate by mass is added, heating, stirring and recrystallizatoin are carried out, drying is carried out after filtering, the mixture is ground and then passes through a screen, and phase-stable ammonium nitrate is obtained, wherein the additive is organic acid sylvine and is selected from potassium formate or potassium acid phthalate or potassium ferricyanide (III) or phthalimide potassium.The preparing method is simple and easy to implement, low in cost, safe in operation and harmless to the human body, and the phase change of ammonium nitrate at 32.1 DEG C (IV-III) and 84 DEG C (III-II) can be inhibited; meanwhile, a small amount of the additive is added, the energy of ammonium nitrate based energy-containing compounds is not influenced, and compared with other ammonium nitrate phase-change materials, phase-stable ammonium nitrate has remarkable advantages.
Owner:内蒙聚力工程爆破有限公司
A kind of synthetic method of S-glycidyl phthalimide
ActiveCN109384773BReduce manufacturing costSuitable for industrial productionOrganic chemistryHydrogen halidePolymer science
The invention relates to a low-cost and high-purity S-glycidyl phthalimide synthesis method, which comprises: carrying out a substitution reaction on potassium phthalimide and R-2,2-disubstituted-4-halomethyl-1,3-dioxolane to generate N-(S-2,2-disubstituted-1,3-dioxolane-4-)methylphthalimide, carrying out a ketone (aldehyde) removing ring-opening reaction to produce N-2-S-hydroxy-3-halogenated n-propyl phthalimide, and finally carrying out an elimination reaction to remove hydrogen halide so as to generate S-glycidyl phthalimide, wherein S-glycidyl phthalimide is the key intermediate for the preparation of rivaroxaban. According to the present invention, the synthesis method has advantages of inexpensive and easily-available raw materials, high stability, high reaction selectivity and highproduction efficiency, and the obtained S-glycidyl phthalimide has advantages of low cost and high purity, and is favorable for the industrial production of high-purity rivaroxaban.
Owner:XINFA PHARMA
A kind of preparation method of phase stable ammonium nitrate
ActiveCN105801323BInhibition of phase transitionDoes not affect energyNon-explosive stabilisersPotassiumPotassium ferricyanide
The invention discloses a preparing method for phase-stable ammonium nitrate.The preparing method comprises the following steps that dried, ground and smashed ammonium nitrate is dissolved in a solvent; a phase stabilizing additive with the adding amount 1-3% of ammonium nitrate by mass is added, heating, stirring and recrystallizatoin are carried out, drying is carried out after filtering, the mixture is ground and then passes through a screen, and phase-stable ammonium nitrate is obtained, wherein the additive is organic acid sylvine and is selected from potassium formate or potassium acid phthalate or potassium ferricyanide (III) or phthalimide potassium.The preparing method is simple and easy to implement, low in cost, safe in operation and harmless to the human body, and the phase change of ammonium nitrate at 32.1 DEG C (IV-III) and 84 DEG C (III-II) can be inhibited; meanwhile, a small amount of the additive is added, the energy of ammonium nitrate based energy-containing compounds is not influenced, and compared with other ammonium nitrate phase-change materials, phase-stable ammonium nitrate has remarkable advantages.
Owner:内蒙聚力工程爆破有限公司
Microwave solid-phase method for synthesizing tetraphenylporphyrinatozinc (II)
ActiveCN109232590ARelaxed response environmentThe reaction conditions are limitedOrganic chemistryPorphyrinSolvent
The invention discloses a microwave solid-phase method for synthesizing tetraphenylporphyrinatozinc (II). The microwave solid-phase method comprises the following preparation steps: (1) weighing malonic acid, potassium phthalimide and zinc acetate, and mixing and grinding to obtain mixed powder; (2) irradiating the mixed powder through microwaves and completely reacting to obtain a reactant; (3) after cooling the reactant in a dark place, carrying out column chromatography separation. The microwave solid-phase method has the beneficial effects that (1) reaction is carried out under a solid phase, reaction environments are wide, the limitation on reaction conditions is small and the practicality is strong; (2) microwave three-dimensional heating reaction is adopted so that the reaction timeis effectively shortened, a reaction energy barrier is reduced, no thermal sublimation occurs and the yield is improved; (3) the reaction does not need a solvent and utilization and recycling of thesolvent can be avoided so that the cost is reduced; (4) no solvent participates in the reaction and purification is facilitated.
Owner:HUANGGANG NORMAL UNIV
Process for preparation of Linezolid and its novel intermediates
A novel process for preparing oxazolidinone antibacterial agent Linezolid including key intermediates of oxazolidinones comprising: reacting 3-fluoro-4-morpholinyl aniline with R-epichlorohydrin; carbonylation to form oxazolidinone derivative; acetylation of (5R)-5-(chloromethyl)-3-(3-fluoro-4-morpholinophenyl-oxazolidin-2-one with sodium acetate to get novel intermediate; hydrolysis of (R)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl methyl acetate; mesylation of (R)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl methanol; reaction of (R)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl methyl methane sulphonate with potassium phthalimide; hydrolysis of (S)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl methyl phthalimide with hydrazine hydrate; acetylation of (S)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl methyl amine with acetic anhydride yields Linezolid in high yield.
Owner:LEE PHARMA LTD
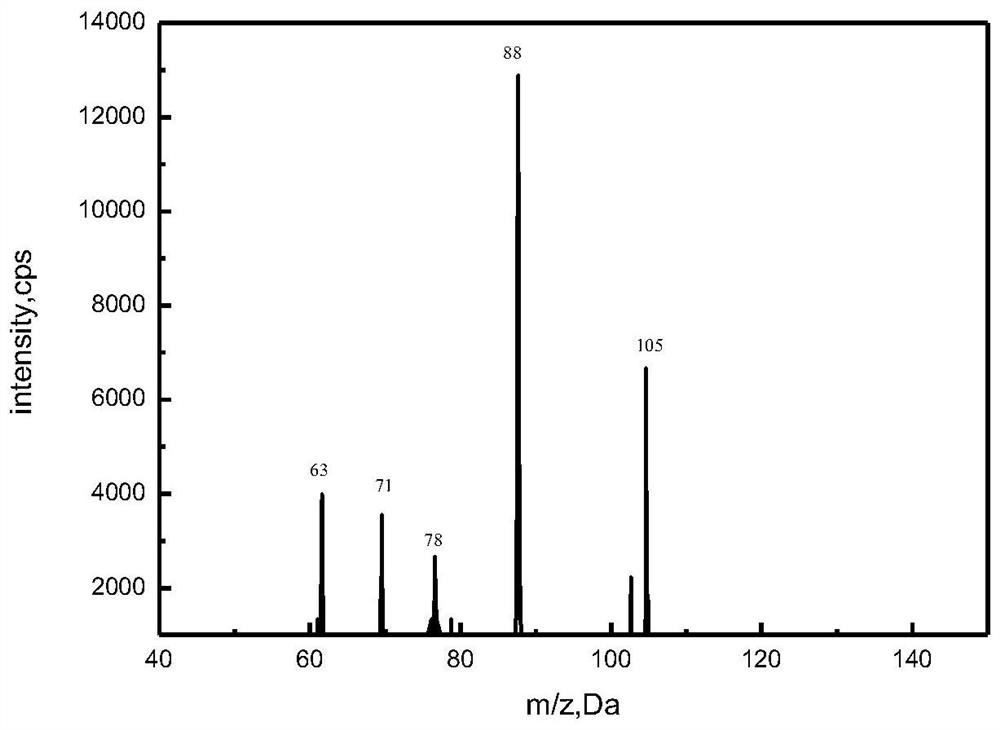
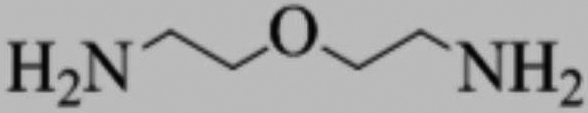
A kind of synthetic method of 2'-oxydiethylamine and product thereof
ActiveCN109988072BAvoid Yield ProblemsPrevent purityOrganic compound preparationAmino-hyroxy compound preparationImideOrganic solvent
The invention discloses a method for synthesizing 2'-oxydiethylamine and its products, which belong to the field of compound preparation. The method uses dichlorodiethyl ether as a starting material, and firstly combines potassium phthalimide with potassium phthalimide in the second place. Heating reaction in an organic solvent to generate 2,2'-diphthalimide diethyl ether, which is purified and reacted with hydrazine hydrate in a second organic solvent by heating to reflux to generate crude product 2'-oxydiethylamine After the crude product 2'-oxydiethylamine is purified, the pure product 2'-oxydiethylamine is obtained; the product yield of this method is high, the purity is high, and the controllability is strong; the product purity obtained is higher than conventional The method has been greatly improved.
Owner:安徽工业大学科技园有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com