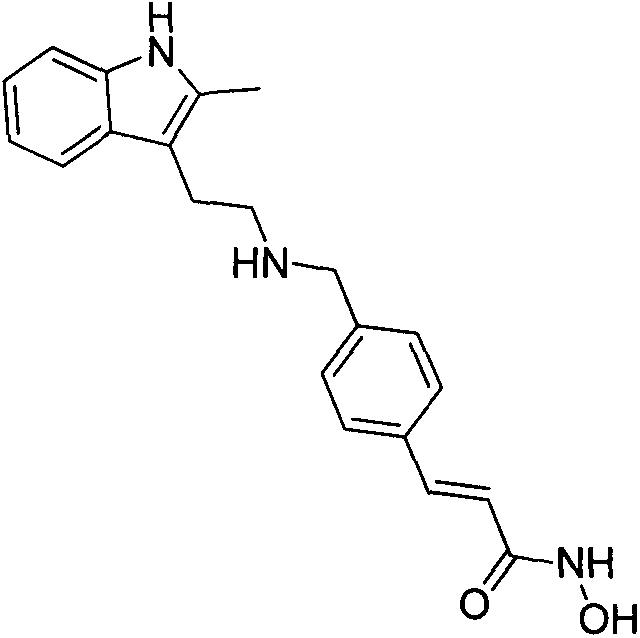
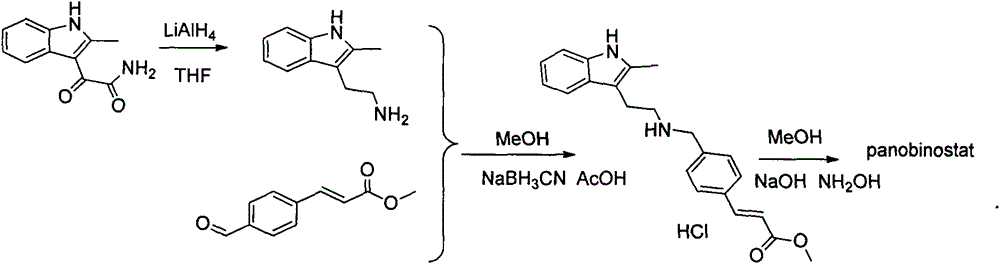
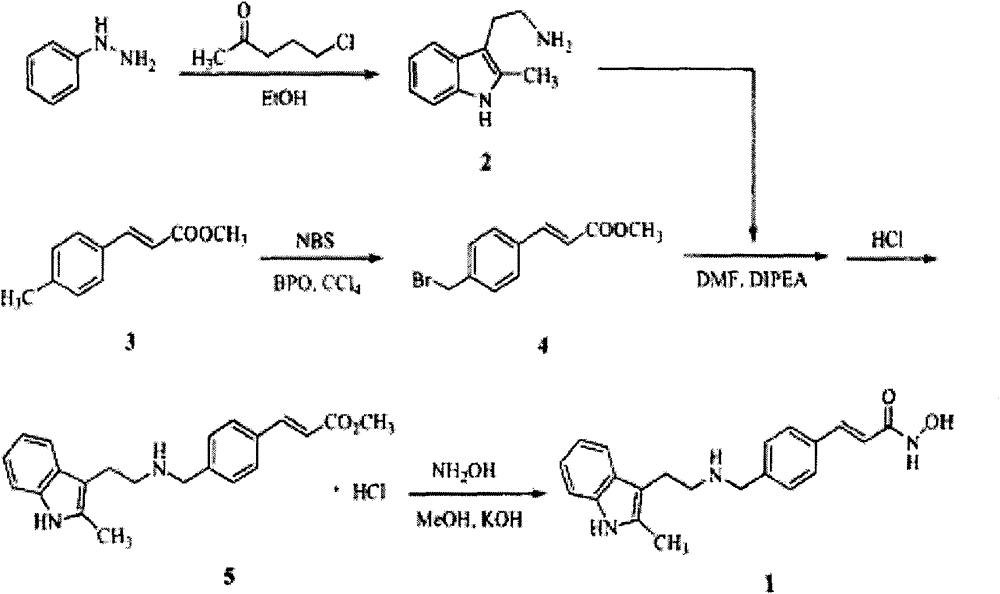
Synthesis method of panobinostat
A synthesis method, the technology of panobinostat, which is applied in the field of panobinostat synthesis, can solve the problems of raw material toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of 2-chloro-1-(2-methyl-1H-indol-3-yl)ethanone
[0061] Add 25g (0.19mol) of 2-methylindole, 250ml of acetonitrile, and 40.9g (0.381mol) of 2,6-lutidine into a 500ml four-necked flask, stir and dissolve at 20-30°C, and control the temperature at 25-35°C. ℃, add 32.3g (0.286mol) of chloroacetyl chloride dropwise, after the dropwise addition is completed, continue to stir for 1 hour, point the plate to detect that the raw materials have basically reacted completely, add 250ml of water dropwise, after fully stirring, suction filter, and use acetonitrile / water=150ml / 150ml The filter cake was rinsed and dried at 50° C. to constant weight to obtain 31.56 g of off-white solid with a yield of 79.7%. Mass: m / z 208.03[M+H] + , mp: 217.3°C.
Embodiment 2
[0063] 2-[2-(2-Methyl-1H-indol-3-yl)-2-oxoethyl]isoindoline-1,3-dione
[0064] Add 2-chloro-1-(2-methyl-1H-indol-3-yl)ethanone solid 10g (0.048mol), DMF40ml, phthalimide potassium 10.6g ( 0.057mol), stir, heat up to 85°C, react for 2 hours, start TLC detection until the basic reaction of raw materials is complete, cool down to 20-30°C, filter with suction, rinse the filter cake with 10ml DMF, merge into the filtrate, add water dropwise to the filtrate 100ml, a solid was precipitated, the temperature was controlled at 20-30°C and fully stirred for 30 minutes, suction filtered, rinsed, and dried at 50°C to constant weight to obtain 13.18g of a light yellow solid with a yield of 86%. Mass: m / z 319.06[M+H] + , mp: 220.1°C-223.5°C.
Embodiment 3
[0066] Preparation of 2-amino-1-(2-methyl-1H-indol-3-yl)ethanone
[0067] Add 2-[2-(2-methyl-1H-indol-3-yl)-2-oxoethyl]isoindoline-1,3-dione solid 6g ( 0.019mol), ethanol 60ml, methylamine aqueous solution (25% by weight-30% by weight) 6g, heat up to 85 degrees, stir for 2 hours, start TLC detection, until the raw materials are basically complete, the reaction solution is prepared to make sand, and column chromatography separates , using pure dichloromethane to pass through the column to obtain 2.8 g of a light yellow solid product with a yield of 79%. Mass: m / z 189.06[M+H] +, mp: greater than 250°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com