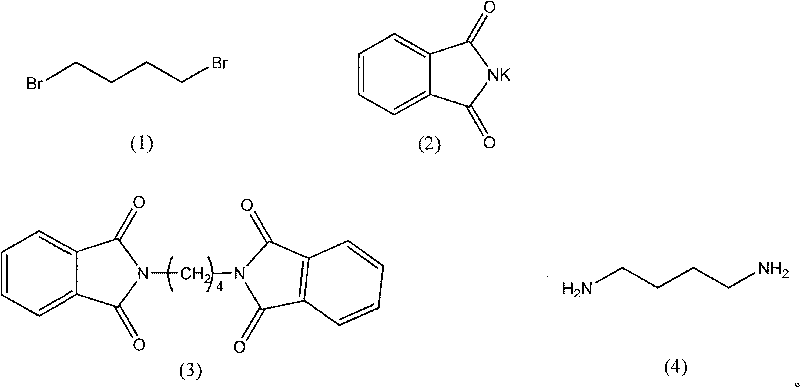
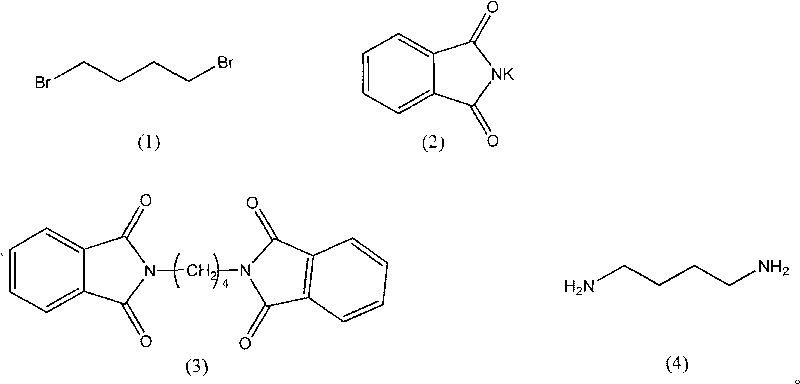
Synthesis method of 1,4-butanediamine
A synthetic method and the technology of butanediamine, which are applied in the field 1, can solve the problems of unsafe industrial production, failure to meet energy saving and emission reduction, and high process operation requirements, and achieve maximum implementation value and social and economic benefits, facilitate industrial production, and process conditions easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 10.90g (0.05mol) of 1,4-dibromobutylamine, add it to a 500ml three-neck flask, add 200mL acetone, stir at 70°C, add 22.20g (0.12mol) potassium phthalimide, and reflux reaction After 14 hours, TLC detected that the reaction reached the end point, and the reaction solution was suction filtered to obtain a white insoluble matter, which was washed twice with 20 ml of distilled water and then dried to obtain 15.66 g of 1,4-diphthalimide butane. 90%, mp.227-229°C. Add 15.66g (0.045mol) of 1,4-diphthalimidebutane prepared in the previous step to a 250mL three-neck flask, add 100mL of ethanol, and then add 18.90g of 40% methylamine aqueous solution (containing methylamine The number of moles of the amine is 0.24 mol), heated until the solid is completely dissolved, and continued to stir until the reaction solution was yellow and transparent, then added 8 mL of concentrated hydrochloric acid, and continued to reflux for 4 hours to end the reaction. The reaction product wa...
Embodiment 2
[0024]Weigh 10.90g (0.05mol) of 1,4-dibromobutylamine, add it to a 500ml three-necked flask, add 200mL of ethanol, stir at 70°C, add 22.20g (0.12mol) of potassium phthalimide, and reflux After 15 hours, TLC detected that the reaction reached the end point, and the reaction solution was suction filtered to obtain a white insoluble matter, which was washed twice with 20 ml of distilled water and then dried to obtain 11.23 g of 1,4-diphthalimide butane. 72.3%, mp.227-229°C. Add 12.58g (0.036mol) of 1,4-diphthalimidebutane prepared in the previous step to a 250mL three-necked flask, add 100mL of ethanol, and then add 16.81g of 40% methylamine aqueous solution (containing The molar weight of methylamine is 0.21mol), heated until the solid is completely dissolved, and continuously stirred until the reaction solution is yellow and transparent, then 8ml of concentrated hydrochloric acid is added, and the reaction is terminated after continuing to reflux for 4 hours. The reaction prod...
Embodiment 3
[0026] Weigh 10.90g (0.05mol) of 1,4-dibromobutylamine, add it to a 500ml three-neck flask, add 200mL methanol, stir at 70°C, add 22.20g (0.12mol) potassium phthalimide, and reflux After 12 hours, TLC detects that the reaction reaches the end point, and the reaction solution is suction filtered to obtain a white insoluble matter, which is washed twice with 20 ml of distilled water and then dried to obtain 11.23 g of 1,4-diphthalimide butane. 64.5%, mp.227-229°C. Add 11.23g (0.032mol) of 1,4-diphthalimidebutane prepared in the previous step to a 250mL three-necked flask, add 100mL of ethanol, and then add 14.99g of 40% methylamine aqueous solution (containing methylamine The molar weight of the amine is 0.19 mol), heated until the solid is completely dissolved, and continuously stirred until the reaction solution is yellow and transparent, then 8ml of concentrated hydrochloric acid is added, and the reaction is terminated after continuing to reflux for 4 hours. The reaction pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com