Method for preparing nitrogen-doped carbon material with high specific surface area from phthalimide potassium
A technology of potassium phthalimide and high specific surface area, which is applied in the direction of carbon preparation/purification, products, reagents, etc., which can solve the problems of complex process costs and other problems, and achieve good adsorption characteristics, high specific surface area, and simplified preparation The effect of craft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation steps of the nitrogen-doped carbon material in this example are as follows: 1 g of potassium phthalimide is placed in a tube furnace, and the temperature of the furnace is raised from room temperature at a rate of 5 °C / min under nitrogen protection (60 ml / min). to 600°C, and heat at 600°C for 1 hour. After natural cooling, the high-temperature-treated product was treated with 10% hydrochloric acid, washed repeatedly with deionized water, and dried at 100° C. for 4 hours to obtain the final product.
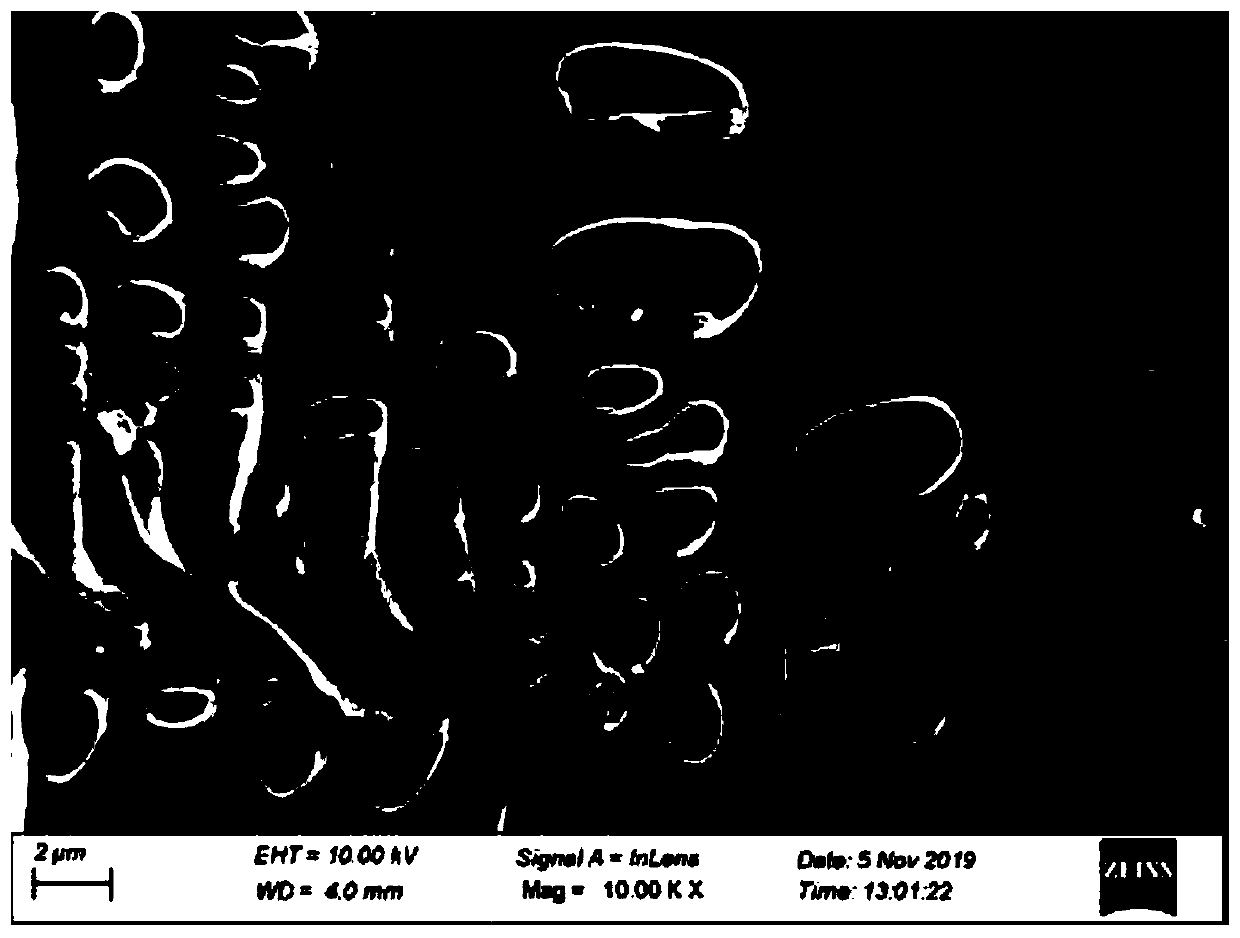
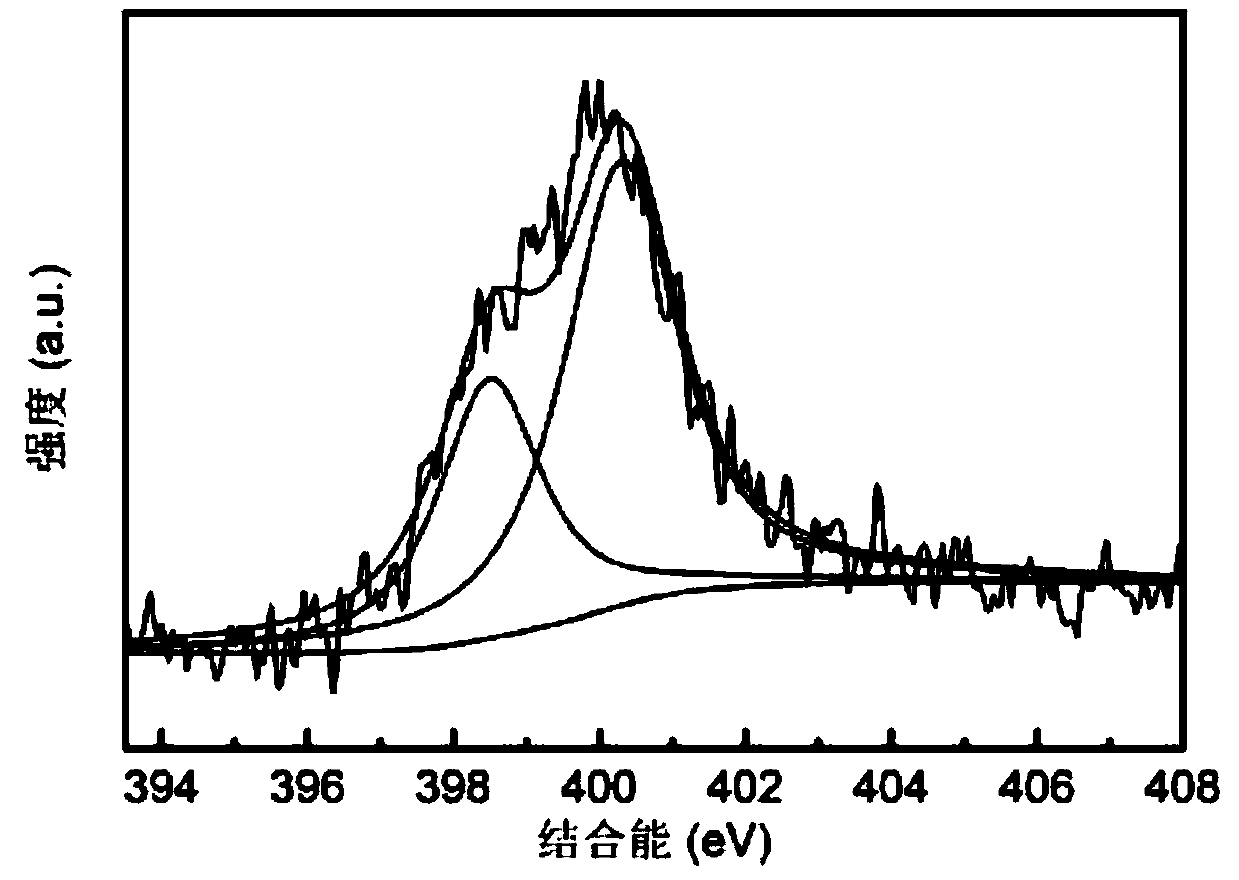
[0041] According to the present embodiment, the nitrogen-doped carbon material scanning electron microscope photo prepared by potassium phthalimide is as follows figure 1 As shown, the carbon material presents irregular large blocks, and large pores of different sizes are formed on the surface. The X-ray photoelectron energy spectrum N1s spectrogram of this carbon material is as follows figure 2 As shown, it is proved that nitrogen can be directly doped i...
Embodiment 2
[0043] The preparation steps of the nitrogen-doped carbon material in this example are as follows: 1 g of potassium phthalimide is placed in a tube furnace, and the temperature of the furnace is raised from room temperature at a rate of 5 °C / min under nitrogen protection (60 ml / min). to 900°C, and heat at 900°C for 2 hours. After natural cooling, the high-temperature-treated product was treated with 10% hydrochloric acid, washed repeatedly with deionized water, and dried at 100° C. for 4 hours to obtain the final product.
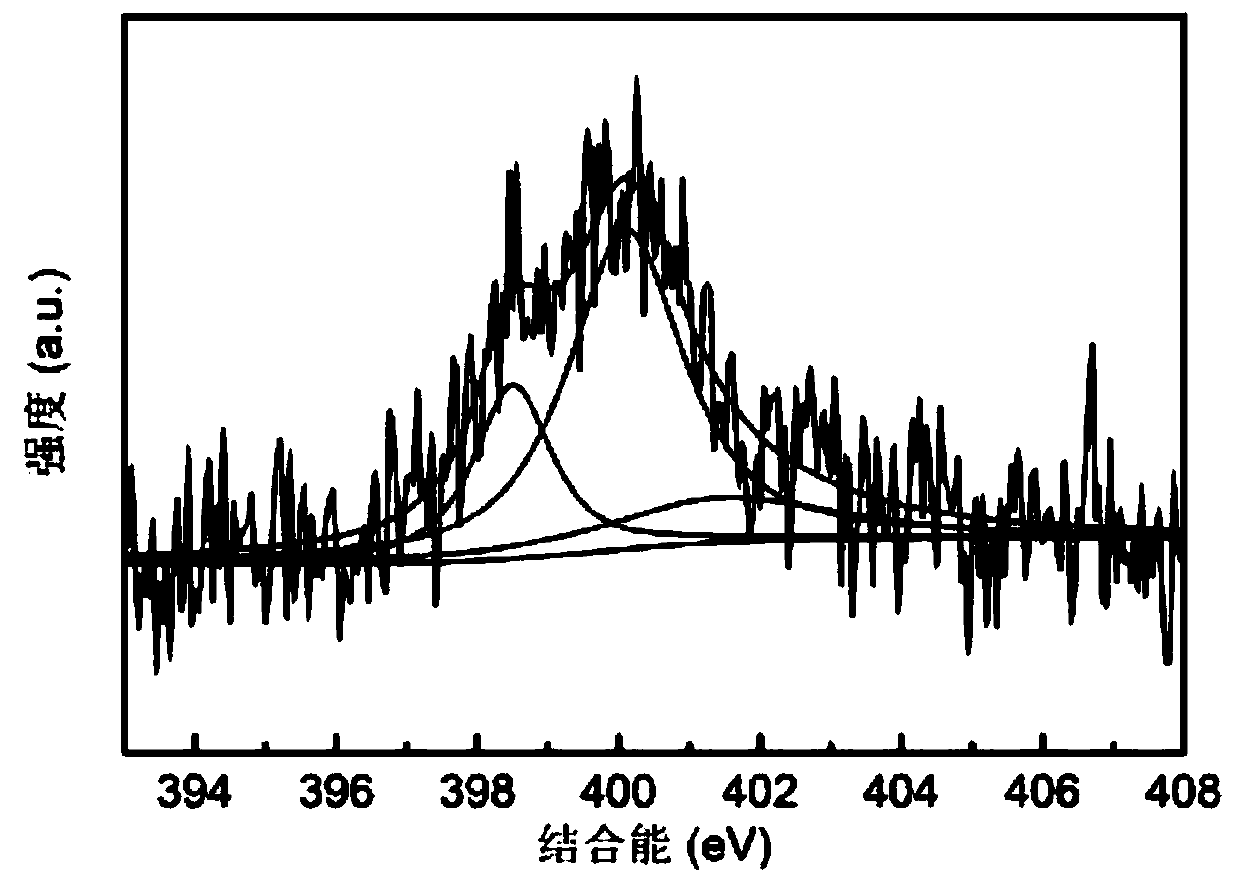
[0044] According to the present embodiment, the nitrogen-doped carbon material X-ray photoelectron energy spectrum N1s spectrogram prepared by potassium phthalimide is as follows image 3 As shown, due to the harsher carbonization conditions, the nitrogen content is reduced to 3.3wt%; the nitrogen adsorption and desorption isotherm diagram of the carbon material at 77K is shown in Figure 4 As shown, its specific surface area is 2053m 2 / g, the pore volum...
Embodiment 3
[0046]The preparation steps of the nitrogen-doped carbon material in this example are as follows: 1 g of potassium phthalimide is placed in a tube furnace, and the temperature of the furnace is raised from room temperature at a rate of 5 °C / min under nitrogen protection (60 ml / min). to 800°C, and heat at 800°C for 1 hour. After natural cooling, the high-temperature-treated product was treated with 10% hydrochloric acid, washed repeatedly with deionized water, and dried at 100° C. for 4 hours to obtain the final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com