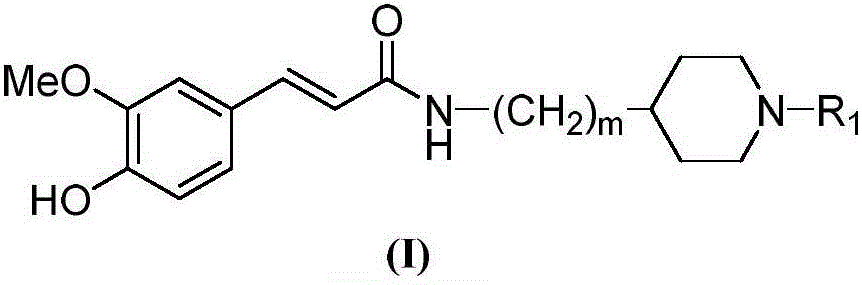
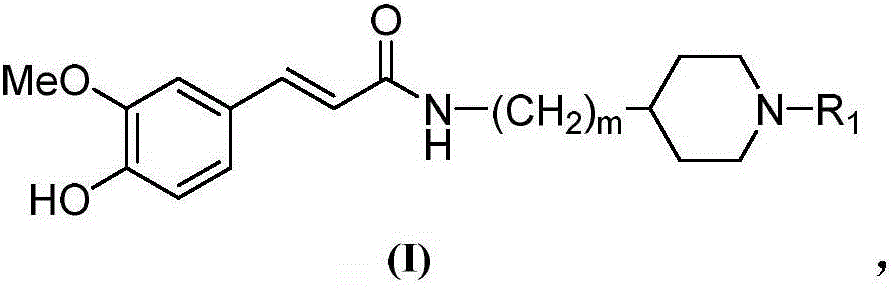
Cyclamine alkylamide ferulate compound as well as preparation method and application thereof
The technology of acid cyclic amine alkyl amide and phthalimide alkyl amine is applied in the field of ferulic acid cyclic amine alkyl amide compound and its preparation, and can solve the problem of single action target, many toxic and side effects, AD patients have problems such as poor long-term efficacy, and achieve the effect of good therapeutic effect, convenient operation and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
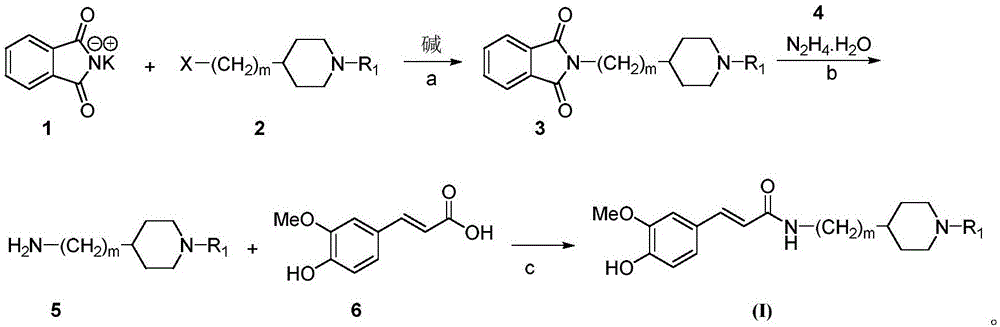
[0041] The preparation method of the ferulic acid cyclic aminoalkyl amides compound of above-mentioned embodiment 1, comprises the following steps:
[0042] a. Add 10mmol phthalimide potassium salt (1), 30mL acetone, 12mmol anhydrous potassium carbonate and 12mmol 1-substituted-4-chloroalkylpiperidine (2) into the reaction flask, heat up to 60°C and reflux Stir the reaction for 20 hours. After the reaction is over, filter while hot, wash the filter cake with a small amount of acetone, evaporate the filtrate to remove the solvent under reduced pressure, and purify the residue by column chromatography (eluent: chloroform) to obtain phthalimide alkane Base amine compound (3), yield is 97.5%;
[0043] b. Dissolve all the above intermediate (3) in 50mL ethanol, add 30mmol N 2 h 4 .H 2 O, heat up to 80° C., reflux and stir for 8 hours. After the reaction, suction filter while hot, wash the filter cake with a small amount of ethanol, evaporate the filtrate to remove the solvent un...
Embodiment 1-60
[0049] Table 1 Embodiments of the present invention 1-60 ferulic acid cyclic amine alkyl amides compounds
[0050]
[0051]
[0052]
[0053] Table 2 One of the parameters of the preparation method of 1-10 ferulic acid cyclic amine alkyl amides of the present invention
[0054]
[0055]
Embodiment 1-10
[0056] Table 3 Embodiment 1-10 of the present invention The second parameter of the preparation method of ferulic acid cyclic amine alkyl amides compound
[0057]
[0058] Table 4 The third parameter of the preparation method of the embodiment of the present invention 1-11 ferulic acid cyclic amine alkyl amides compound
[0059]
[0060]
[0061] Preparation of Ferulic Acid Cyclic Amino Alkyl Amide Compound (I) and Acid Synthetic Salt
[0062] Add 2.0mmol of ferulic acid cyclic amine alkyl amides compound (I) and 50ml of acetone prepared according to the above-mentioned embodiment 1 in the reaction flask, add 6.0mmol tartaric acid after stirring evenly, heat up and reflux stirring reaction for 20min, cool to At room temperature, the solvent is evaporated under reduced pressure, the residue is recrystallized with acetone, and the precipitated solid is filtered to obtain the salt of ferulic acid cyclic aminoalkylamide compound (I). 1 Confirmed by HNR and ESI-MS.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com