A kind of synthetic method of S-glycidyl phthalimide
A technology of glycerol phthalimide and potassium phthalimide, applied in the field of medicine and biochemical industry, can solve problems such as unfavorable industrialized production, long reaction time, difficult aftertreatment, etc., and the reaction temperature is not harsh , the effect of high reaction yield and purity, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
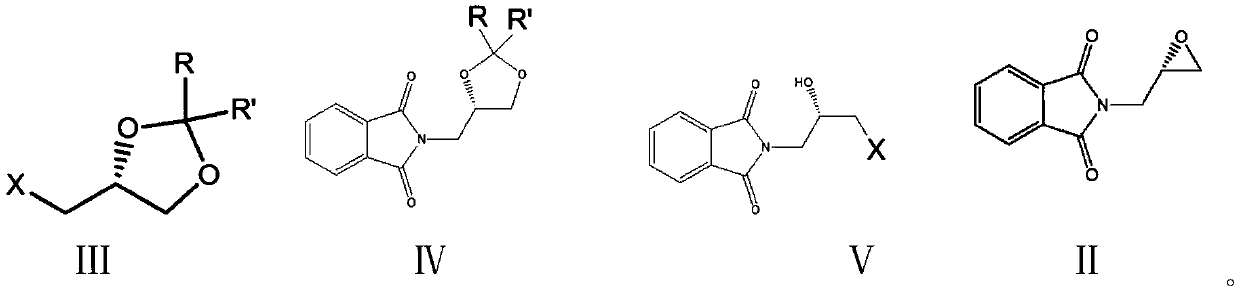
[0058] Example 1: N-(S-2,2-dimethyl-1,3-dioxolane-4-)methylphthalimide (IV 1 ) preparation
[0059] Add 250 grams of N,N-dimethylformamide (DMF), 37.0 grams (0.20 moles) of potassium phthalimide, 33.3 grams of (0.22 mol) R-2,2-dimethyl-4-chloromethyl-1,3-dioxolane, 0.5 g of potassium iodide, heated, and kept stirring at an internal temperature of 80-85°C for 4 hours. Cool to 20°C, filter, wash the filter cake with 50 g of DMF, and combine the filtrates. DMF was recovered by distillation under reduced pressure, and the residue was recrystallized with 80 g of methyl tert-butyl ether to obtain 49.7 g of N-(S-2,2-dimethyl-1,3-dioxolane-4-)methyl-o- Phthalimide, yield 95.3%, HPLC purity 99.7%.
[0060] The preparation process of embodiment 2-embodiment 5 is the same as embodiment 1, and difference list 1 is as follows:
[0061] Table 1: Example 2-Example 5:
[0062]
Embodiment 6
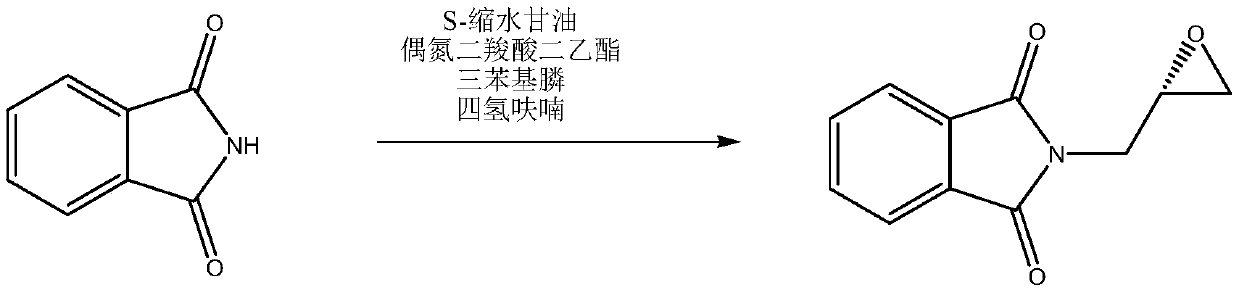
[0069] Embodiment 6: Preparation of S-glycidylphthalimide (II)
[0070] Add 200 g of 1,2-dichloroethane, 26.5 g (0.10 moles) of N-(S-2,2-dimethyl-1,3-di Oxolane-4-)methylphthalimide, 25 grams of 40% hydrobromic acid, stirred and reacted at 10-15°C for 4 hours. The layers were separated, and the aqueous layer was extracted three times with 1,2-dichloroethane, 20 g each time, the organic phases were combined, and the organic phases were transferred to a constant-pressure low-liquid funnel. In another flask, add 30 g (0.15 moles) of 27% sodium methoxide methanol solution, keep the internal temperature between 0-5°C and add the obtained organic phase dropwise, after dropping, stir and react at 10-15°C for 3 hours. Add 200 grams of ice water, separate layers, extract the water layer twice with 1,2-dichloroethane, 20 grams each time, combine the organic phases, recover 1,2-dichloroethane by distillation, and use 50 grams of methanol to extract the residue. tert-butyl ether was rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com