Thioether allyl isothiocyanate compounds, and preparation method and applications thereof
A technology for horseradish and compounds, which is applied in the fields of chemical synthesis and biological activity research to achieve the effects of good inhibition effect, obvious killing effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention provides the preparation method of above-mentioned thioether horseradish, comprising the following steps:
[0040] S1) Dissolve the alkane substituted at both ends in an organic solvent, then add potassium phthalimide and a phase transfer catalyst, heat to 50-80°C, react for 1-8 hours, perform TLC tracking experiment, and then evaporate the solvent , washed with water, and recrystallized from methanol or ethanol to obtain intermediate 1: N-(2-bromoethyl)phthalimide;
[0041] S2) Dissolve intermediate 1 in an organic solvent, add sodium hydrosulfide and a phase transfer catalyst, protect with inert gas, react at 10-50°C for 0.5-12 hours, perform TLC follow-up experiment, wash with water, extract with an organic solvent, and saturated saline After washing and evaporating the solvent, the intermediate 2 was obtained by column chromatography;
[0042] S3) Intermediate 3 is prepared from intermediate 2: a thioether compound. Intermediate 2 reacts with ...
Embodiment 1
[0055] Embodiment 1, intermediate 1: the preparation of N-(2-bromoethyl) phthalic acid imide:
[0056]
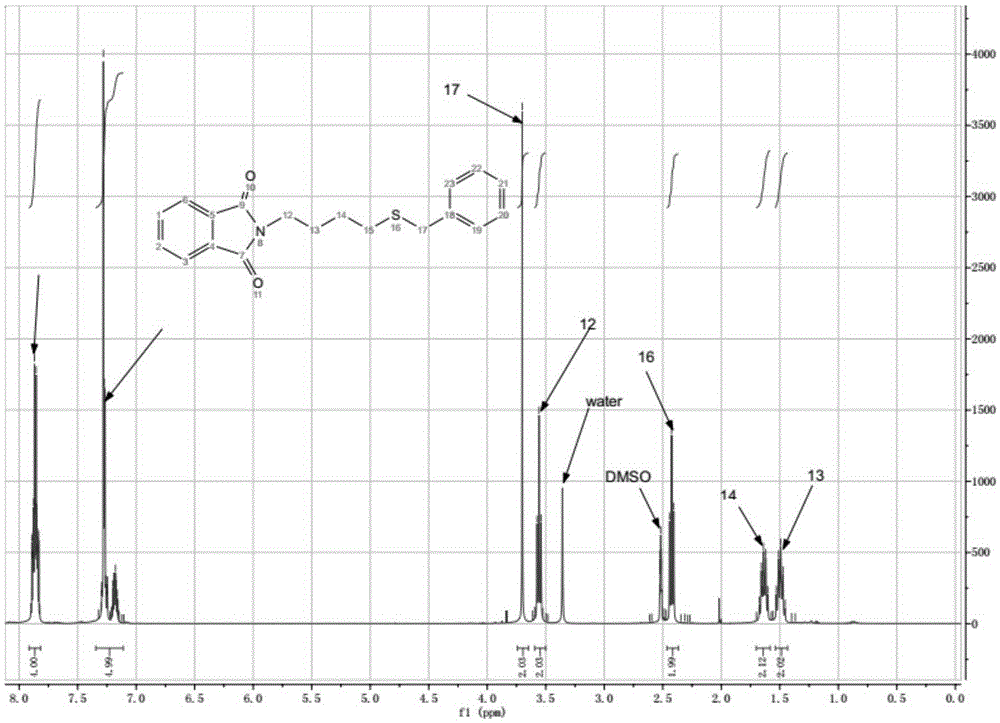
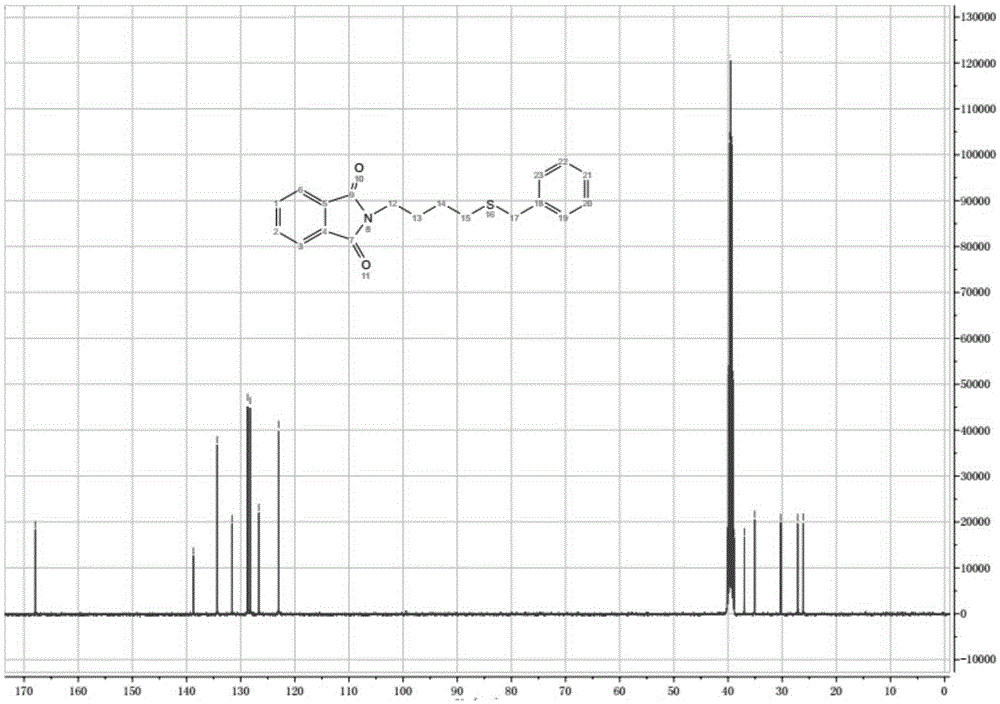
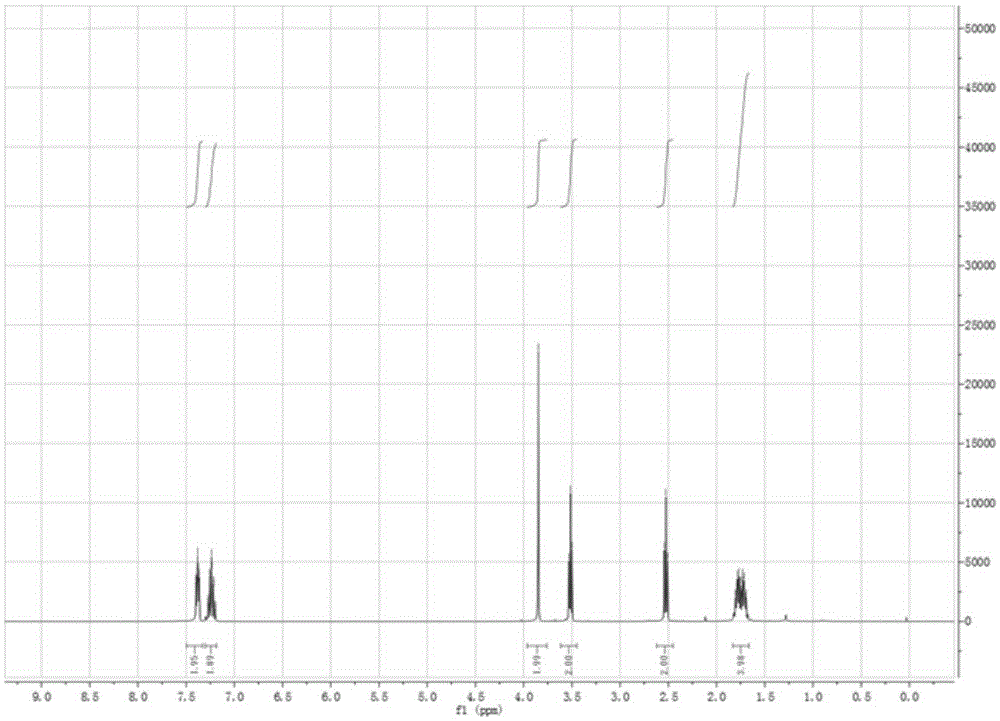
[0057] In a 250 mL two-necked flask, 50 mmol of phthalimide, 150 mmol of 1,2-dibromoethane, 2.0 g of TBAB (tetrabutylammonium bromide) phase transfer catalyst and 120 mL of DMF (dimethylformamide) were sequentially added. Heating to 50-80°C, heating and reacting for 5h, TLC monitoring [developing solvent, [V (petroleum ether): V (acetate) = 5:1] until the end of the reaction. The solvent was distilled off under reduced pressure, and the residue was added to ice water. A large amount of white solid appeared, which was suction filtered, washed, and dried. The obtained solid was transferred to a 250 mL dry flask, and 30 mL of methanol was added for recrystallization. After cooling, crystallization, suction filtration, and drying, 10.3 g of a white solid was obtained, with a yield of 81% and a melting point of 75-76°C; 1 HNMR (CDCl 3 ,400MHz), δ:3.62(t,J=6.7Hz,2H),4.11(t,J...
Embodiment 2
[0059] Embodiment two, intermediate 2: the preparation of mercaptan:
[0060]
[0061] The synthesis of mercaptans has been reported in many domestic and foreign literatures. Among them, U.S. Patent US20130210704 reports that bromide reacts with thiourea first, and then undergoes alkalization to prepare mercaptans; in addition, international patent application WO2012082436 and Chinese patent CN201210296425.2 report bromine The compound is reacted with potassium thioacetate to prepare thioester compound, and then acidic hydrolysis is used to prepare mercaptan, and the total yield is less than 60%. The synthesis steps of mercaptans in the prior art are all two-step synthesis. In the present invention, bromide and sodium hydrosulfide are used to prepare mercaptans in one step, which has the advantages of simple process and high yield.
[0062] In a 100 mL two-necked flask, 5 mmol of N-(2-bromoethyl) phthalimide, 6 mmol of sodium hydrosulfide, 0.30 g of TBAB and 60 mL of DMF we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com