Process for preparing 5-aminolevulinic acid from 5-chloromethyl furfural
A technology of aminolevulinic acid and chloromethylfurfural, which is used in the preparation of cyanide reaction, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions and dangerous reaction process, and achieves mild reaction conditions, The effect of high reaction yield and selectivity, good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
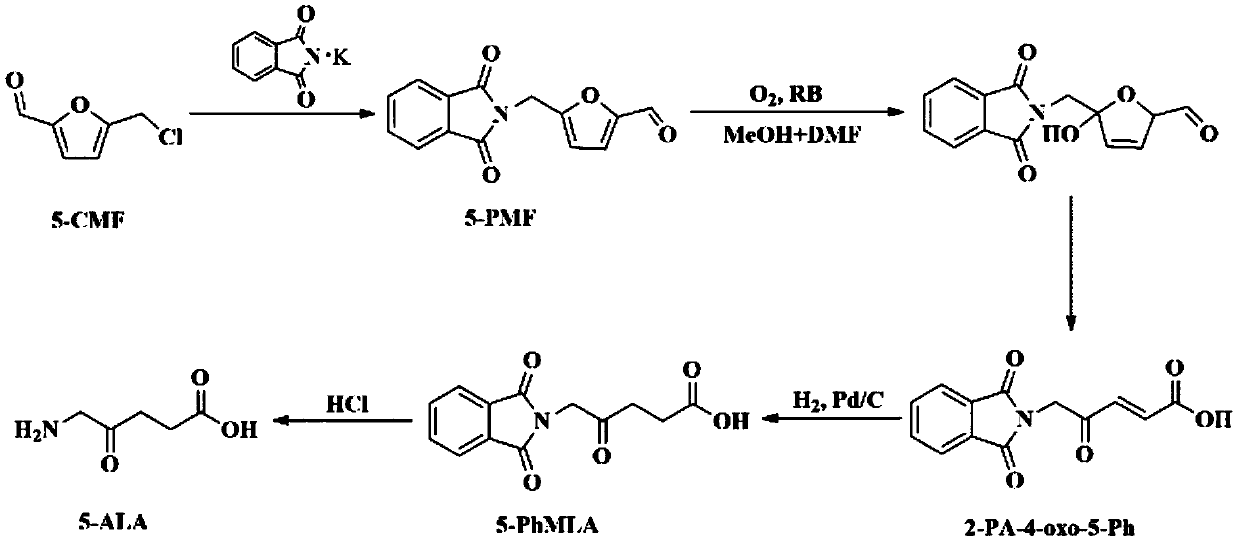
[0026] A kind of technique of preparing 5-aminolevulinic acid with 5-chloromethylfurfural of the present embodiment comprises the following steps successively:
[0027] (1) Gabriel reaction
[0028] Put 87mg of 5-CMF, 185mg of KPI, and 20mL of DMF in a round-bottomed flask and mix them evenly. React for 4 hours at 20°C. After the reaction, filter and collect the filtrate. Add deionized water at 4°C to the filtrate and let it stand After 30 min, the solid was collected by filtration, and the yield of 5-PMF was measured to be 86.6%.
[0029] (2) Photooxidative ring-opening reaction
[0030] Put 2.3g of 5-PMF, 180mg of Rose Bengal (RB), and 120mL of pyridine in a round bottom flask, with a 250W metal halide lamp providing light, an oxygen flow rate of 0.05L / min, a reaction temperature of 20°C, and a reaction time of 6h After the reaction, the reaction solution was constant to 120mL with pyridine, and the remaining amount of 5-PMF was detected, and the conversion rate of 5-PMF w...
Embodiment 2
[0037] (1) Gabriel reaction
[0038] Put 102mg of 5-CMF, 185mg of KPI, and 20mL of DMF in a round-bottomed flask and mix them evenly. React for 4 hours at 20°C. After the reaction, filter and collect the filtrate. Add deionized water at 4°C to the filtrate and let it stand After 30 min, the solid was collected by filtration, and the yield of 5-PMF was measured to be 89.4%.
[0039] (2) Photooxidative ring-opening reaction
[0040] Put 2.3g of 5-PMF, 120mg of Rose Bengal (RB), and 120mL of acetone in a round-bottomed flask, with a 250W metal halide lamp providing light, an oxygen flow rate of 0.05L / min, a reaction temperature of 20°C, and a reaction time of 6h After the reaction, the reaction solution was constant to 120mL with acetone, and the remaining amount of 5-PMF was detected, and the conversion rate of 5-PMF was measured to be 75.5%. Activated carbon was added to the reaction solution and left to stand for 3 hours for decolorization, the activated carbon was removed b...
Embodiment 3
[0046] (1) Gabriel reaction
[0047] Put 102mg of 5-CMF, 185mg of KPI, and 20mL of DMF in a round-bottomed flask and mix them evenly. React for 3 hours at 120°C. After the reaction, filter and collect the filtrate. Add deionized water at 4°C to the filtrate and let it stand After 30 minutes, the solid was collected by filtration, and the yield of 5-PMF was measured to be 85.3%.
[0048] (2) Photooxidative ring-opening reaction
[0049] 2.3g 5-PMF, 180mg Rose Bengal (RB), 120mL V DMF :V 甲醇 (1:1), placed in a round-bottomed flask, 60W magnesium lamp provides light, oxygen flow rate is 0.05L / min, reaction temperature is 20°C, reaction time is 6h, after the reaction, dilute the reaction solution to 120mL with acetone , Detect the remaining amount of 5-PMF, and the conversion rate of 5-PMF is measured to be 6.2%. Activated carbon was added to the reaction solution and left to stand for 3 hours for decolorization, the activated carbon was removed by filtration, and the solvent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com