Method for preparing sofosbuvir intermediate
A technology for intermediates and compounds, applied in the field of drug synthesis, can solve problems such as high cost and many side reactions, and achieve the effects of low cost, few side reactions and simple handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
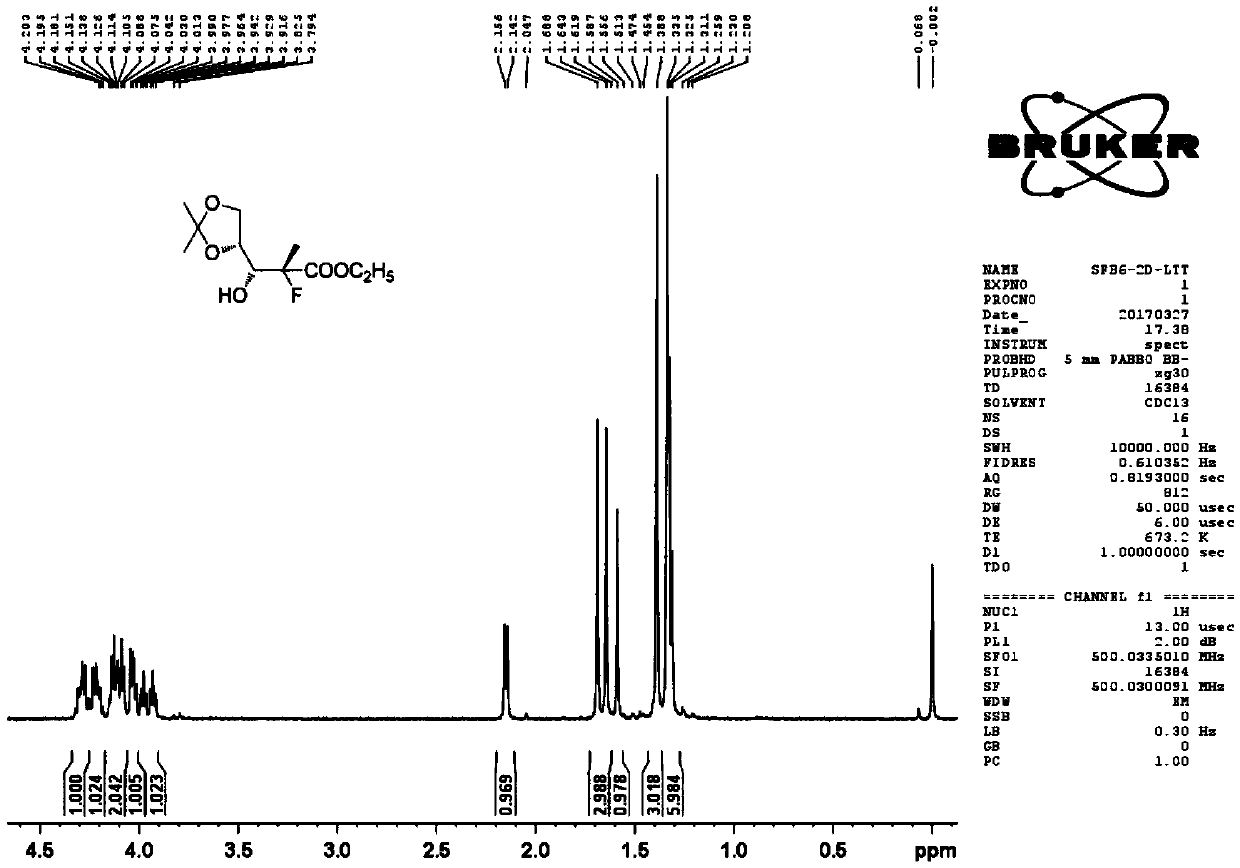
[0041] Take 2-C-methyl-4,S-O-(1-methylethylene)-D-ethyl arabinonic acid cyclic sulfate and 4-phenyl-2-oxazolidinone according to the molar ratio of 1:1.5 , add toluene to react, the reaction temperature is 60°C, and the reaction time is 12 hours. After the reaction, the temperature was lowered to 20° C., washed twice with saturated brine, and the organic phases were combined and concentrated to obtain a yellow oil (the first compound) with a yield of 90%, which was directly used in the next reaction. The reaction formula is as follows:
[0042]
[0043] Add the first compound, tetraethylammonium fluoride, and anhydrous 1,4-dioxane in the reaction flask, the molar ratio of the first compound and tetraethylammonium fluoride is 1:2.0, and the resulting reaction mixture is heated to 105°C, the solvent was refluxed, and the reaction was stirred for 16 hours. After TLC (thin-layer chromatography) monitors the complete reaction of the raw materials, the reaction mixture is coole...
Embodiment 2
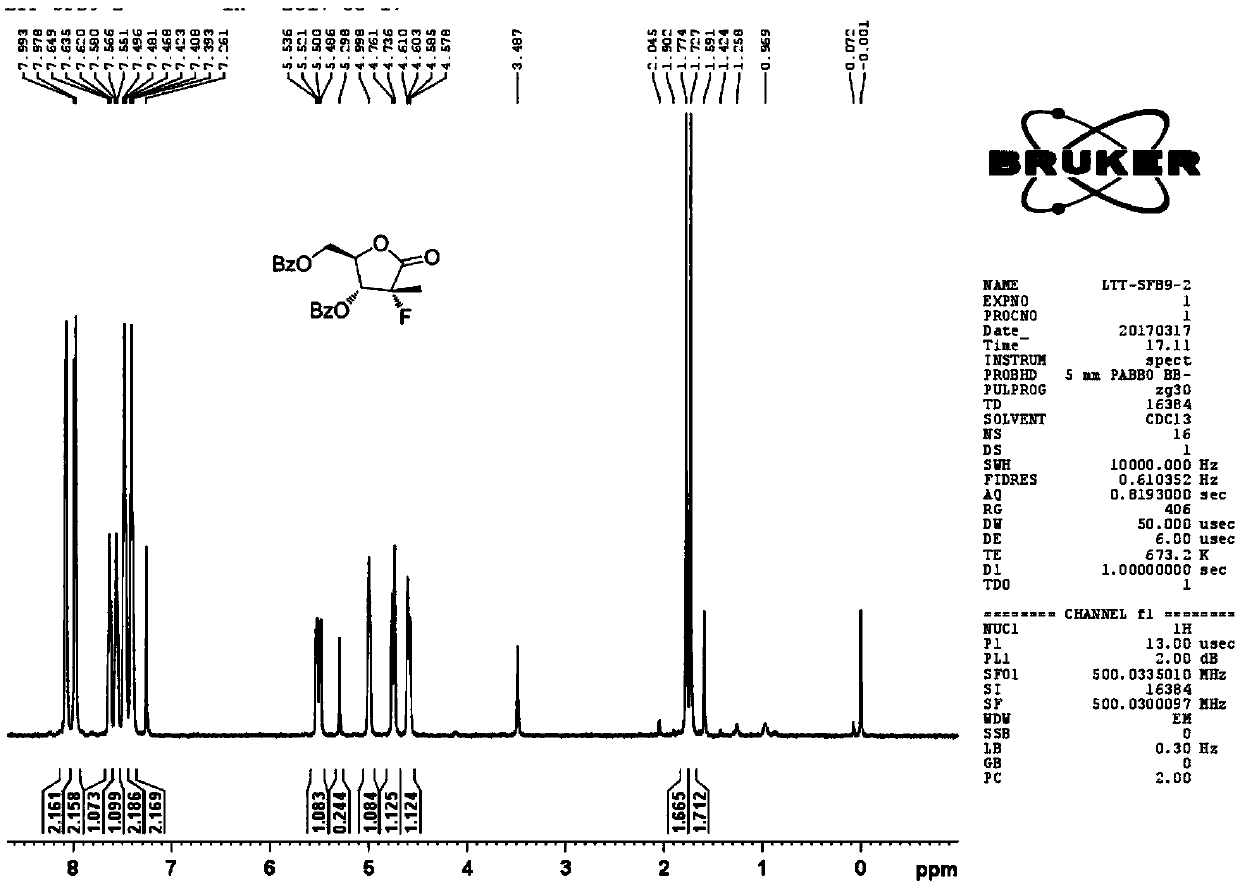
[0050] Take 2-C-methyl-4,S-O-(1-methylethylene)-D-ethyl arabinonic acid cyclic sulfate and 4-phenyl-2-oxazolidinone according to the molar ratio of 1:2.0 , add toluene to react, the reaction temperature is 80°C, and the reaction time is 8 hours. After the reaction, the temperature was lowered to 30° C., washed twice with saturated brine, and the organic phases were combined and concentrated to obtain a yellow oil (the first compound) with a yield of 95%, which was directly used in the next reaction.
[0051] Add the first compound, tetraethylammonium fluoride, and anhydrous 1,4-dioxane in the reaction flask, the molar ratio of the first compound and tetraethylammonium fluoride is 1:2.0, and the resulting reaction mixture is heated to 115°C, the solvent was refluxed, and the reaction was stirred for 18 hours. After TLC (thin-layer chromatography) monitors the complete reaction of the raw materials, the reaction mixture is cooled to room temperature, and 2,2-dimethoxypropane (1...
Embodiment 3
[0055] Take 2-C-methyl-4,S-O-(1-methylethylene)-D-ethyl arabinonic acid cyclic sulfate and 4-phenyl-2-oxazolidinone according to the molar ratio of 1:1.8 , add toluene to react, the reaction temperature is 70°C, and the reaction time is 10 hours. After the reaction, the temperature was lowered to 25° C., washed twice with saturated brine, and the organic phases were combined and concentrated to obtain a yellow oil (the first compound) with a yield of 92%, which was directly used in the next reaction.
[0056] Add the first compound, tetraethylammonium fluoride, and anhydrous 1,4-dioxane in the reaction flask, the molar ratio of the first compound and tetraethylammonium fluoride is 1:1.9, and the resulting reaction mixture is heated to 110°C, the solvent was refluxed, and the reaction was stirred for 17 hours. After TLC (thin-layer chromatography) monitors the complete reaction of the raw materials, the reaction mixture is cooled to room temperature, and 2,2-dimethoxypropane (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com