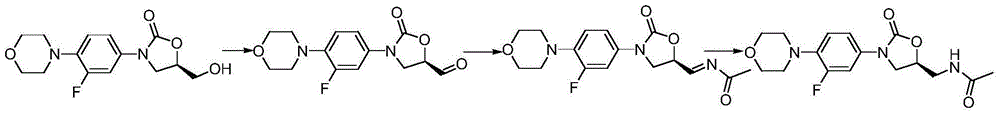
Linezolid preparation method
A technology of linezolid and oxazolidinone, applied in the field of preparation of linezolid, can solve the problems of harsh reaction conditions, expensive raw materials, easy moisture absorption of anhydrous lithium tert-butoxide and the like, and achieves mild reaction conditions and environmental protection. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In a 1000ml reaction flask, add 200ml of dichloromethane, (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-hydroxymethyl-2-oxazolidinone 40g , 2 grams of 20% potassium bromide aqueous solution, cooled to -5 ℃, add catalyst tetramethylpiperidine nitrogen oxide 0.4g under stirring, dropwise add mass percentage concentration and be 400 grams of sodium hypochlorite solution of 6.5%, after dropwise addition , reacted at 0°C for 1 hour, added dichloromethane for extraction, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the solvent to obtain (5R)-3-[3-fluoro-4-(4-olino)phenyl ]-5-formaldehyde-2-oxazolidinone crude product 36.5 g, yield 91.8%. Dissolve 36.5 g of the crude product of (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-formaldehyde-2-oxazolidinone in 500ml of methanol, raise the temperature to 20°C, and slowly add ethyl alcohol 7.3 g of amide was followed by liquid chromatography. After the reaction was completed, 4.7 g of sodium borohydride was directl...
Embodiment 2
[0016]In a 1000ml reaction flask, add 200ml of dichloromethane, (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-hydroxymethyl-2-oxazolidinone 20 grams , 10 grams of 20% potassium bromide aqueous solution, be cooled to 5 ℃, add catalyzer tetramethylpiperidine nitrogen oxide 2g under stirring, dropwise mass percent concentration is 400 grams of sodium hypochlorite solution of 6.5%, after dropwise addition, 10 ℃ for 3 hours, adding dichloromethane for extraction, drying the organic phase with anhydrous sodium sulfate, filtering, and concentrating the solvent to obtain (5R)-3-[3-fluoro-4-(4-olyl)phenyl]- The crude product of 5-formaldehyde-2-oxazolidinone was 15.6 grams, and the yield was 78.5%. Dissolve 15.6 g of the crude product of (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-formaldehyde-2-oxazolidinone in 500ml of methanol, raise the temperature to 50°C, and slowly add ethyl 4.7 g of amide was followed by liquid chromatography. After the reaction was completed, 4.3 g of potassium borohydride w...
Embodiment 3
[0018] Add 200ml of dichloromethane and 30 grams of (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-hydroxymethyl-2-oxazolidinone into a 1000ml reaction flask , 10 grams of 20% potassium bromide aqueous solution, be cooled to 2 ℃, add catalyzer tetramethyl piperidine nitrogen oxide 2g under stirring, dropwise mass percentage concentration is 400 grams of sodium hypochlorite solution of 6.5%, after dropwise addition, 5 ℃ for 2 hours, added dichloromethane for extraction, the organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was concentrated to obtain (5R)-3-[3-fluoro-4-(4-olyl)phenyl]- The crude product of 5-formaldehyde-2-oxazolidinone was 25.7 grams, and the yield was 86.2%. Dissolve 25.7 g of the crude product of (5R)-3-[3-fluoro-4-(4-olyl)phenyl]-5-formaldehyde-2-oxazolidinone in 500ml of methanol, raise the temperature to 30°C, and slowly add ethanol 7.0 g of amide was tracked by liquid chromatography. After the reaction, 6.5 g of potassium borohydride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com