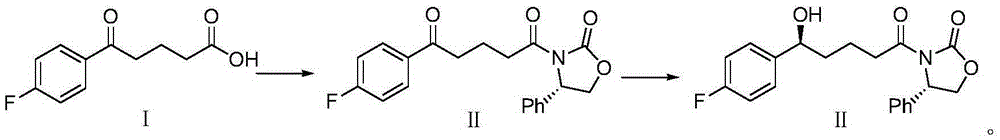
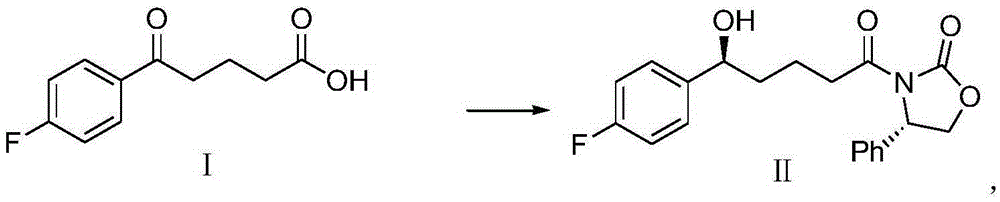
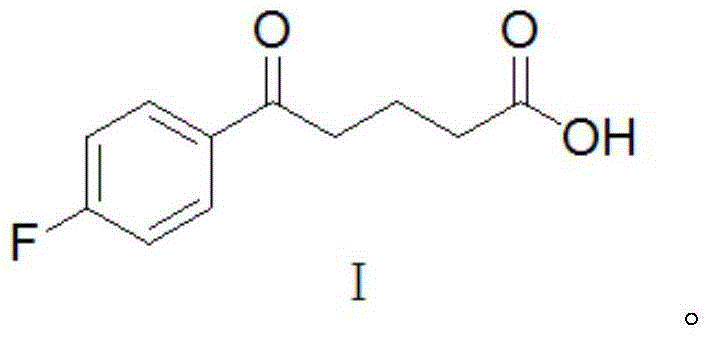
Synthetic method for ezetimibe intermediate
A synthetic method, ezetimibe technology, applied in the direction of organic chemistry, can solve the problems of long synthetic route and high synthetic cost, and achieve the effect of low synthetic cost, short synthetic route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be described in detail below in conjunction with various embodiments, but it should be noted that these embodiments are not limitations of the present invention, and those of ordinary skill in the art can make functional, method, or structural equivalent transformations or replacements based on these embodiments. , all fall within the protection scope of the present invention.
[0020] Unless otherwise specified in the description, the components and raw materials in each example of the present invention are of analytical grade. In addition, "g" in each embodiment is the weight unit "gram"; "h" is the time unit "hour"; "ml" is the volume unit "milliliter"; "room temperature" is 23°C; Liquid Chromatographic Detection". "Concentration" is mass percentage.
[0021] The present invention adopts "one-pot method" to synthesize ezetimibe intermediate (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1, 3-Oxazolane-2-one (II) has the adv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com