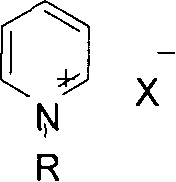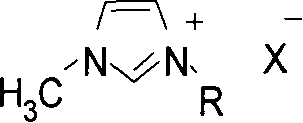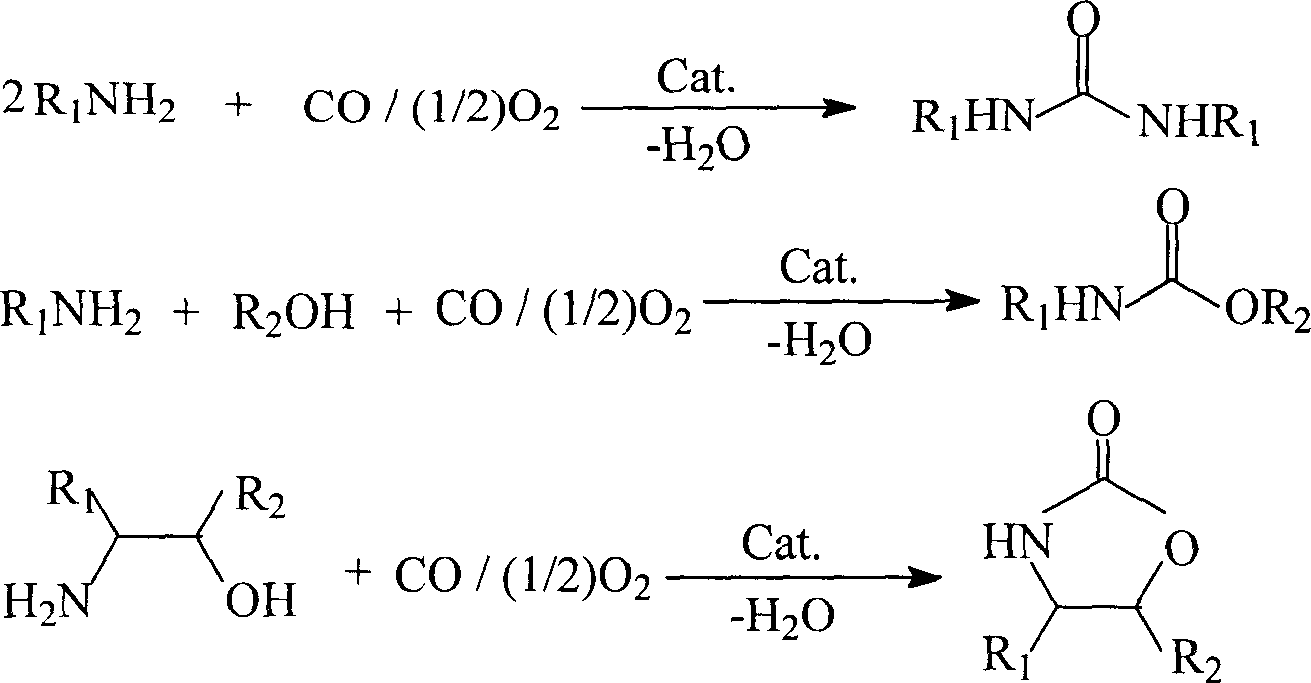Process for preparing carbamate, urea and their derivatives as well as 2-oxzolidone
A technology of carbamate and oxazolidinone is applied in the field of preparing nitrogen-containing carbonyl compounds, which can solve the problems of equipment corrosion, difficulty, and increase the cost of catalyst use, and achieves mild reaction conditions, high product selectivity, and catalyst system. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] In a 100 milliliter autoclave, add palladium acetate 0.005mmol and cocatalyst 1,3-dimethyl-imidazolium iodide (MMImI) 0.02mmol successively, then add 50mmol amine compounds and 10 milliliters of ethylene glycol dimethylamine reaction solvent , airtight reaction kettle; the pressure of filling oxygen is 0.5Mpa and the pressure of carbon monoxide is 3.5MPa, and reacting for a certain period of time under the condition of reaction temperature of 120°C. After the reaction, cool to room temperature and unload the kettle; the reaction solution precipitates white needle-shaped product N, N'-diphenylurea (2a) at low temperature, and is filtered and weighed to calculate the yield. After qualitative and quantitative analysis by 6890 / 5973 chromatography-mass spectrometry, high-performance liquid chromatography, and nuclear magnetic resonance, the purity of the product is greater than 99%, and the separation yield can reach 96%.
Embodiment 2
[0034] The cocatalyst used is 1-methyl-3-butyl-imidazolium iodide (BMImI), and the remaining reaction conditions are the same as in Example 1; the isolated yield is 98%, and the selectivity is greater than 99%.
Embodiment 3
[0036]The metal catalyst used is palladium carbon, and the remaining reaction conditions are the same as in Example 1; the separation yield is 95%, and the selectivity is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com